|
|
|
|
 |
 |
| |
 |
|
|
Original Articles |
|
|
|
|
|
Keywords :
renal Doppler, liver cirrhosis, hepatorenal syndrome, ascites |
|
|
Yasser M Fouad1, Hamdy Mokarrab1, Ahmed Fathi Elgebaly2, Hussein El Amin4, Ehab M Abdel Raheem1, Mohamed A Sharawy3, Mohamed E Shatat3
Department of Tropical Medicine &Gastroenterology1, Radiology2, andInternal Medicine3,
Minya University,
Minya and Departmnet of Internal Medicine,
Gastroenterology Unit4,
Assuit University,
Assuit,Egypt.
Corresponding Author:
Dr. Yasser M Fouad
Email: yasserfouad10@yahoo.com
DOI:
http://dx.doi.org/
Abstract
Aim: To study the renal resistive index (RI) and pulsatility index (PI) measured by renalDoppler in various stages of liver cirrhosis and their values to detect cirrhotic patients at risk for developing the hepatorenal syndrome
Methods: This study included 60 cirrhotic patients divided into 4 groups (15 patients each):compensated liver cirrhosis (group A) , diuretic responsive ascites (group B), refractoryascites (group C) , hepatorenal syndrome (group D) and ten healthy persons as the controlgroup (E). All patients were subjected to detailed history taking and clinical examination.Laboratory investigations included simple urine analysis, complete blood picture, liver functiontests, blood urea and serum creatinine, serum sodium and serum potassium, 24-hoururine collection for sodium concentration, creatinine concentration and protein concentration.Ultrasonographic examination and renal duplex Doppler ultrasonography were undertakento assess the RI and PI
Results: The RI of both interlobar and arcuate arteries was significantly higher in all patientgroups than in the control group (p‹0.01). The RI was significantly higher in patients withrefractory ascites than in patients with diuretic responsive ascites, and also in patients withdiuretic responsive ascites than in patients with compensated cirrhosis (p‹0.01); in patientswith hepatorenal syndrome than in patients with diuretic responsive ascites and patientswith compensated cirrhosis (p‹0.0001). The PI was significantly higher in all patients groupsthan in the control group (p‹0.01) and in patients with refractory ascites than in patients withdiuretic responsive ascites and was also higher in patients with responsive ascites than inpatients with compensated cirrhosis (p‹0.0001). Also, the PI was significantly higher in patientswith hepatorenal syndrome than in patients with responsive ascites and patients withcompensated cirrhosis (p‹0.0001). Creatinine clearance in patients with the hepatorenalsyndrome was significantly lower than that of other different groups (p<0.0001) but there wasno significant change in creatinine clearance between patients with compensated cirrhosisand control group. While creatinine clearance in patients with diuretic responsive asciteswas significantly higher than that in patients with compensated cirrhosis (p<0.05) there wasno significant change between patients with diuretic responsive ascites and patients withrefractory ascites
Conclusion: Both renal resistive index and pulsatility index increase with the degree of hepaticdecompensation. Renal duplex ultrasound which is a non-invasive, simple and easy methodto study intrarenal hemodynamics in patients with liver cirrhosis may predict patients at riskof hepatorenal impairment.
|
48uep6bbphidvals|171 48uep6bbphidcol4|ID 48uep6bbph|2000F98CTab_Articles|Fulltext Renal hemodynamic changes with intense intrarenalvasoconstriction begin early in the course of liver diseasebefore changes in the level of serum urea and serumcreatinine.[1] Patients with liver cirrhosis and portal hypertensiondevelop circulatory dysfunction characterised by disturbancein systemic and renal hemodynamics. Hepatorenal syndrome(HRS) is a “functional” and reversible form of renal failure that occurs in patients with advanced chronic liver disease. Thedistinctive hallmark feature of HRS is the intense renalvasoconstriction caused by interactions between systemicand portal hemodynamics. This results in activation ofvasoconstrictors and suppression of vasodilators in the renalcirculation. Although the assessment of kidney function is ofgreat clinical importance in patients with liver cirrhosis and ascites, serum creatinine and even creatinine clearance arenot accurate indicators of renal impairment in patients withadvanced liver cirrhosis. Most patients with hepatorenalsyndrome do not have a satisfactory response to diuretictherapy.[2]
Doppler ultrasonography is a non-invasive tool for theassessment of vascular patency and has been used tomeasure hepatic arterial and venous flow in patients with portalhypertension. Although, a diagnostic grey scaleultrasonography is widely employed in the evaluation of cirrhoticpatients, and Doppler is rarely used.[3] Time velocity waveformanalysis of Doppler signals from small intrarenal arteriesallows for estimation of intrarenal vascular resistance.Resistive index (RI) is the most widely used index for estimationof intrarenal vascular resistance.[4]
An elevated renal RI has been observed in variousconditions associated with elevated renal vascular resistancelike kidney obstruction, acute tubular necrosis and hemolyticuremic syndrome and is detected in liver disease relatedfunctional kidney failure.[5] Kastelan et al, have demonstratedthat renal duplex Doppler of interlobar arteries is a simple,effective and non-invasive method and enables the earlydetection of renal hemodynamic disturbances in patients withliver cirrhosis even before renal dysfunction becomes clinicallyevident. Moreover it makes possible the identification of asubgroup of patients with liver cirrhosis who are at higher riskfor developing HRS.[6]
Sacerdoti et al, have found that renal arterial resistanceindices (PI and RI) evaluated by duplex ultrasonographyincrease in the non-ascitic phase of cirrhosis and are higherin the ascitic phase, particularly during diuretic treatment. Thisnon-invasive method may be applied to pathophysiologicaland clinical studies of the renal functional impairment ofcirrhosis.[7]
Methods
This study was performed by the Tropical Medicine Departmentin conjunction with the Radiology Department, Minia UniversityHospital during the period of January to September 2008. Thestudy included 60 cirrhotic patients, seropositive for HCV, invarious stages, randomly selected from those admitted to theTropical Medicine Department in Minia University Hospital.The patients were divided into 4 groups:
Group A included 15 patients with compensated livercirrhosis, Group B included 15 patients with diuretic responsiveascites, Group C: 15 patients with refractory ascites and GroupD: 15 patients suffering from hepatorenal syndrome diagnosedclinically and by renal chemistry, and 10 healthy persons takenas a control group (Group E).
This work has been carried out in accordance with theDeclaration of Helsinki (2000) of the World Medical Association.All patients provided informed written consent.
All patients were subjected to clinical assessment includingdetailed history of chronic liver disease especially bleedingtendency, ascites, jaundice, and encephalopathy. Patientswere excluded if there was a history of diabetes mellitus,hypertension, and nephrotoxic drug intake.
Laboratory investigations included simple urine analysis,complete blood picture, liver function tests including total anddirect bilirubin, ALT, AST, alkaline phosphatase, total proteins,serum albumin and prothrombin time and concentration,fasting and post-prandial blood glucose level, blood urea andserum creatinine, serum sodium and serum potassium, 24-hour urine collection for sodium concentration, creatinineconcentration and protein concentration was done. Creatinineclearance: the urine was collected for 24 hours, urine andserum creatinine levels were measured, and the clearancerate was then calculated:
Creatinine Clearance (mL / min) = (Urine C / Serum C ) x(Urine Volume (mL) / (time (hour) x 60)
Ultrasonographic examination was performed on all patientsand the control group at the Radiology Department in MinyaUniversity Hospital. The equipment used was MedisoneSonoace 9900 Duplex ultrasonography equipment with acurved convex 3.5-5 MHz transducer with a real time B modeimaging system with pulsed wave and colour Doppler facilities.All subjects were studied in the morning after overnight fasting.They underwent A- Abdominal and Pelvic Ultrasonography:The liver, spleen, kidneys, and ascites were evaluated and B-Renal duplex doppler ultrasonography: to assess dopplerindices.
Patients were examined in the supine position as well as inthe right and left lateral positions. The classic midline approachof the origin of the main renal arteries was done by placing thetransducer in the epigastrium with slight inclination to the leftto visualize the abdominal aorta in a coronal section with itsmain abdominal branches (celiac and superior mesentericarteries). This was followed by a cross-sectional view of theabdominal aorta just distal to the origin of the superiormesenteric artery in order to visualise the renal arteries. Theorigin of the two renal arteries was determined by two importantlandmarks (i.e. the superior mesenteric artery and the renalvein). The peak systolic velocity of the aorta was measured.The sample volume was placed with an angle of insinuation(the angle between the incident sample volume and the vessel)between 30ºº and 60º to obtain an accurate and optimumvelocity recording avoiding the high errors obtained with biggerangles. Waveform recording was first obtained from theabdominal aorta just proximal to the origin of the renal arteries.Recordings were obtained a few centimetres from the originof the aorta to avoid high velocity turbulence at the origin. Therenal artery was then traced down to the hilum of thecorresponding kidney. The patient was placed on the rightlateral or left lateral position to obtain a good view of theintrarenal branches inside the corresponding kidney. Thefollowing parameters were calculated from each interlobarartery and the arcuate artery:
-
Peak systolic velocity, which is the peak of the systolicwaveform
-
End diastolic velocity, which is the velocity at the end of thediastolic phase
-
Mean velocity, which is the velocity throughout thecardiac cycle
-
Resistive and pulsatility index, calculated according tospecial software fitted to the computer of the machineMedisone Sonoace 9900 Duplex ultrasonographyequipment. Figures 1, 2 and 3 show representative colourdoppler images in different study groups.
Statistical analysis
Data were checked, coded, entered and analysed using SPSS(The Statistical Package for Social Sciences) version 10.0 software
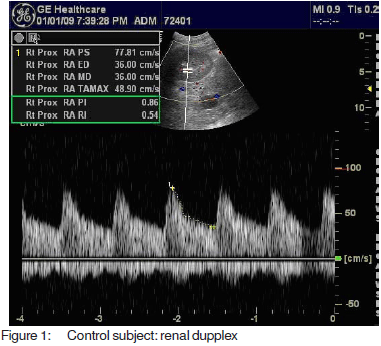
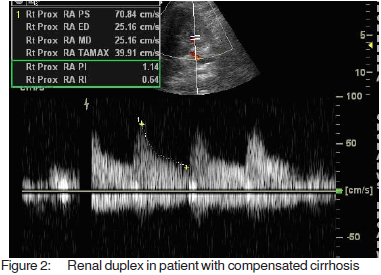
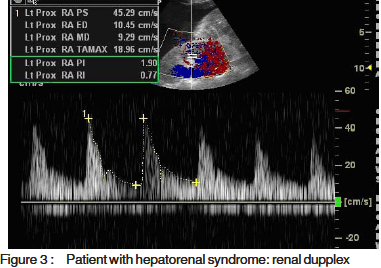
The results were collected, presented and analysedusing the 0.05 significance level and the 0.01 high significancelevel, p value of <0.05 was considered significant. Statisticalmethods included:
-
descriptive methods such as mean, standard deviation,frequency distribution (minimal and maximal), independentt-test, one-way ANOVA,
-
quantitative data to test the significance of differencesbetween the mean values of the study variables forcomparison between more than two groups and Pearsoncorrelation,
-
for determination of the correlation between the age, andsex of different groups and the resistive index and thecorrelation between urinary sodium and the resistive indexin different groups.
Results
The study included 60 cirrhotic patients, selected from thoseadmitted to the Tropical Medicine Department in MiniaUniversity Hospital. There were 35 male and 25 femalepatients with age ranging from 30 to 65 years. Demographicdata are shown in Table 1. There was no significant differencebetween all groups regarding age and sex.
With note to the liver function tests, prothrombinconcentration and serum albumin were significantly higher inpatients with compensated liver cirrhosis than in patients withhepatorenal syndrome while serum bilirubin was significantlylower in patients with compensated liver cirrhosis than inpatients with the hepatorenal syndrome (p<0.05) (Table 2). There were no significant changes in prothrombinconcentration, serum albumin and serum bilirubin betweenpatients with diuretic responsive ascites, patients withrefractory ascites and patients with hepatorenal syndromeThere were no significant changes in prothrombinconcentration, serum albumin and serum bilirubin betweenpatients with diuretic responsive ascites, patients withrefractory ascites and patients with hepatorenal syndrome(Table 2).
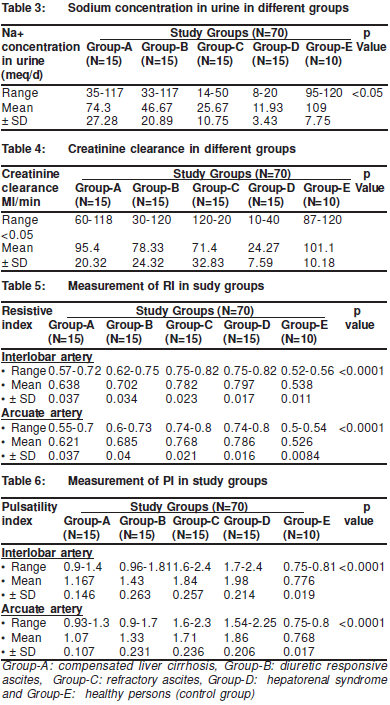
The mean urinary sodium concentration was significantlyhigher in Group-A than in Group-B. It was also higher inGroup -B than in Group-C patients and in Group-C patientsthan that in Group-D patients but was lower in Group-A thanthat of Control Group-E (Table 3).
The Creatinine clearance in patients with hepatorenalsyndrome was significantly lower than that of the other groups(p<0.05). But there was no significant change in creatinineclearance between patients with compensated cirrhosis andthe control group. Whilst creatinine clearance in patients withdiuretic responsive ascites was significantly higher than thatin patients with compensated cirrhosis (p<0.05). But therewas no significant difference in the creatinine clearancebetween patients with diuretic responsive ascites and patientswith refractory ascites (Table 4).
Renal doppler RI measurement: the RI of both theinterlobar and arcuate arteries was significantly higher in allpatients groups than in thecontrol group (p‹0.0001). The RI of both interlobar and arcuate arteries was significantly higher in patients with refractory ascites than in patients with diureticresponsive ascites and was also is higher in patients withdiuretic responsive ascites than in patients with compensatedcirrhosis (p‹0.0001). Also, the RI of both interlobar and arcuatearteries was significantly higher in patients with thehepatorenal syndrome than in patients from the other groups(p‹0.0001) (Table 5).
The PI of both interlobar and arcuate arteries wassignificantly higher in all patient groups than in the controlgroup (p‹0.0001). It was also significantly higher in patientswith refractory ascites than in patients with diuretic responsiveascites and in in patients with responsive ascites than inpatients with compensated cirrhosis (p‹0.0001)). Also, the PIof both interlobar and arcuate arteries was significantly higherin patients with hepatorenal syndrome than in patients fromthe other groups (p‹0.0001) (Table 6).
Discussion
Hepatorenal syndrome (HRS) is reversible functional renalimpairment that occurs in patients with advanced liver cirrhosisor those with fulminant hepatic failure. It is characterised bymarked reduction in the glomerular filtration rate (GFR) andrenal plasma flow in the absence of other causes of renalfailure.[8]
The hallmark of HRS is hypoperfusion of the kidneyresulting from combined renal vasoconstriction anddecreased total renal blood flow.[9] The earliest stages of thisapparently functional form of kidney failure often gounrecognised because creatinine elevation occurs late in thehepatorenal syndrome.[10]
Duplex ultrasonography is a widely used non-invasivemethod to assess vascular patency and blood flow in manysites. Duplex Doppler can be used to assess vascularresistance in the small intraparenchymal vessels throughsimple analysis of Doppler waveform by a parameter termedthe resistive index (RI).[11] An elevated renal RI has beenobserved in various conditions associate with elevated renalvascular resistance such as kidney obstruction, acute tubularnecrosis and hemolytic uremic syndrome and should bedetected in liver disease related functional kidney failure.[5]
Our aim was to study the renal resistive index and pulsatilityindex of both interlobar and arcuate arteries in different stagesof liver cirrhosis. Early identification of changes in the RI andPI in patients with established liver disease who are atparticular risk for the development of hepatorenal syndromemay be beneficial in directing or modifying the management. This study was carried out on 60 cirrhotic patients dividedinto 4 groups: Group A (compensated cirrhosis), Group B(responsive ascites), Group C (refractory ascites) and GroupD (hepatorenal syndrome) and a Group-E (control) group.We observed a significant fall in the urinary sodiumconcentration (meq/dL) in the patient groups. Kenawi et al,reported that the urine sodium excretion in patients with chronicliver disease decreases with progression of disease andcorrelates with the Child-Pugh’s class.[12]
We determined that creatinine clearance in the hepatorenalsyndrome group was significantly lower than that of all othergroups but that there was no significant change between the control group and the group with compensated liver cirrhosis,and between the group with responsive ascites and that ofrefractory ascites. Creatinine clearance is less accurate andless sensitive in detecting a group at particular risk fordevelopment of hepatorenal syndrome as it overestimatesthe GFR.[13]
Renal duplex Doppler ultrasonography was performed onthe right or left kidney at the interlobar and arcuate arteries tomeasure the resistive index and the pulsatility index. In thisstudy we found a significant increase in the renal resistiveindex and in the pulsatility index of both interlobar and arcuatearteries among all patient groups. The RI and PI of patientswith diuretic responsive ascites were higher than those ofpatients with compensated cirrhosis without ascites. Also, RIand PI of patients with refractory ascites were higher thanthose of responsive ascites, whilst there were no significantchange in either RI or PI between the refractory and hepatorenalgroups. The increase in renal vascular RI in cirrhotic patientswith ascites can be explained by a physiological homeostaticresponse to vascular underfilling occurring in ascitic patients.When the vascular underfilling is moderate, the renal vasoactivesubstances are effectively counterbalanced by increased renalsynthesis of prostaglandins so that renal blood flow and GFRremain normal. In contrast, when the vascular underfilling issevere, intense stimulation of endogenous vasoconstrictorsystems occurs, producing renal vasoconstriction andimpairment of renal blood flow and GFR.[14] Pateron et al,concluded that intra-renal blood flow is preserved in cirrhoticpatients by intra-renal mechanisms until the ascites becomesrefractory. When this regulation fails renal ischemia causestubular necrosis, azotemia and oliguric renal failure.[15]
Maroto et al, demonstrated that RI is significantly higher indecompensated cirrhotic patients with ascites than incompensated cirrhotic patients and that the RI of compensatedcirrhotic patients is higher than in the controls. They reportedthat these results were highly sensitive and specific for thediagnosis of HRS.[1] Also Masahiko et al, demonstrated thatboth the pulsatility and resistive indices were significantlyhigher in cirrhotic patients compared to controls and comparedto patients with chronic hepatitis. Both indices measured bycolour Doppler ultrasonography were closely related to theseverity of cirrhosis and showed significant correlation withincreased Child-Pugh grade.[16] In another study Alberto et al,concluded that patients with HRS had significantly highervalues of RI than those without HRS. The relative risk ofdeveloping HRS in patients with an RI = 0.70 was high. RI is auseful indicator in patients with cirrhosis and ascites for thediagnosis and prognosis of HRS.[17]
Kastelan et al, demonstrated that renal duplex Doppler ofinterlobar arteries was a simple effective and non-invasivemethod that enables the early detection of renal hemodynamicdisturbances in patients with liver cirrhosis even before renaldysfunction becomes clinically evident and makes possiblethe identification of a subgroup of patients with liver cirrhosiswho are at higher risk for developing the hepatorenalsyndrome.[6] In our study we found that the RI and PI weresignificantly different between patient groups even in theabsence of significant changes in creatinine clearance.
In Italy, Sacerdoti et al, reported that the PI and RI weresignificantly higher in nonascitic cirrhotic patients than incontrol patients, in ascitic patients than in nonascitic patients,in ascitic patients not treated with diuretics than in nonasciticones and in ascitic patients treated with diuretics than in those not treated. The PI and RI were significantly higher in Child-Turcotte-Pugh class B and C patients than in class A patients.This noninvasive method may be applied to pathophysiologicaland clinical studies of the renal functional impairment incirrhosis.[7]
In Mexico, Lopez et al, 2006 found that the RI and PI werehigher in patients with cirrhosis at the time to be comparedwith healthy controls, which represents a diminution in bloodflow. But, they suggested that both indices are higher in cirrhoticpatients. The differences in PI and RI were evident betweeneach Child class.[18]
In this study, patients with diabetes mellitus or hypertensionwere excluded because of the impact of these diseases onrenal hemodynamics and hence the duplex indices.[19,20] Severalstudies have shown that a normal mean renal RI isapproximately 0.60. The largest series to date (58 patients)reported a mean (±SD) RI of 0.60 ± 0.01 for subjects withoutpreexisting renal disease.[21] The normal mean PI isapproximately 0.96 ± 0.10 for healthy subjects.[22]
We conclude that renal duplex Doppler ultrasound is usefulas a non-invasive method for the evaluation of the renalhemodynamic changes in cirrhotic patients with goodcorrelation to the severity of liver disease. RI and PI may besuperior to creatinine clearance as an indicator in patientswith liver cirrhosis for the detection of patients at risk for thedevelopment of HRS.
References
-
Maroto A, Ginès A, Saló J, Clària J, Ginès P, Anibarro L, et al.Diagnosis of functional renal failure of cirrhosis with Dopplersonography: prognostic value of resistive index. Hepatology.1994;20:839–44.
-
Arroyo V, Gines P, Gerbes A, Dudley FJ, Gentilini P, Laffi G, et al.Definition and diagnostic criteria of refractory ascites andhepatorenal syndrome in cirrhosis. Hepatology. 1996;23:164–76.
-
Lomas DJ, Britton PD, Summerton CB, Seymour CA. Duplex Dopplermeasurements of portal vein in portal hypertension. Clin Radiol.1993;48:311–5.
-
Platt JF, RubinJM and Eills JH. Intrarenal arterial Doppler sonographyin patients with non-obstructive renal disease: correlation ofresistive index with biopsy findings. Am J Roentgenol.1990;154:1223–7.
-
Friedman SL. Alcoholic liver disease, cirrhosis, and its majorsequelae In:Cecil text book of medicine; L Goldman, C Bennet, 21st ed Saunders, Philadelphia; 2000,153:804–12.
-
Kastelan S, Ljubicic N, Kastelan Z, Ostojic R, Uravic M. The role ofduplex-doppler ultrasonography in the diagnosis of renaldysfunction and hepatorenal syndrome in patients with livercirrhosis. Hepatogastroenterology. 2004;51:1408–12.
-
Sacerdoti D, Bolognesi M, Merkel C, Angeli P, Gatta A. Renalvasoconstriction in cirrhosis evaluated by duplex Dopplerultrasonography. Hepatology. 1993;17:219-24.
-
Angeli P, Merkel C. Pathogenesis and management of hepatorenalsyndrome in patients with cirrhosis. J Hepatol. 2008;48:S93–103
-
Cárdenas A, Uriz J, Ginès P, Arroyo V. Hepatorenal syndrome. Liver Transpl. 2000;6:S63–71.
-
Gentilini P, Laffi G. Renal function impairment and sodium retentionin liver cirrhosis. Digestion. 1989;43:1–32.
-
Platt JF. Duplex Doppler evaluation of native kidney dysfunction:obstructive and non obstructive disease. AJR Am J Roentgenol.1992;158:1035–42.
-
Kenawi AM, Hamid AA, Sahar A Atty. Renal resistive index andsodium concentration as a predictor of renal dysfunction in patientswith advanced liver cirrhosis. MD thesis, Cairo university 2004.
-
Nix DE, Erstad BL, Nakazato PZ, Barletta JF, Matthias KR, KruegerTS. Estimation of creatinine clearance in end-stage liver disease. Ann Pharmacother. 2006;40:900–8.
-
Abuelo GJ. Diagnosing vascular causes of renal failure. Ann InternMed 1995,123:601–14.
-
Pateron D, Barriere E, Condat B, Rassiat E, Heller J, Chagneau C,et al. relationship between haemodynamic alternations and thedevelopment of ascites or refaractory ascites in patients withcirrhosis. Eur J Gastroentrol Hepatol. 2001;13:251–6.
-
Masahiko K, Murawaki Y, Kawasaki H. Renovascular resistanceassessed by color Doppler ultrasonography in patients with chronicliver diseases Western Pacific Helicobacter Congress No3,pasar,Bali, INDONESIE, 2000, vol. 15, no 12, pp. H1-H33 (19ref.), pp. 1424–1429
-
Bardi A, Sapunar J, Oksenberg D, Poniachik J, Fernández M,Paolinelli P, et al. Intrarenal arterial doppler ultrasonography incirrhotic patients with ascites, with and without hepatorenalsyndrome. Rev Med Chil. 2002;130:173-80.
-
López Méndez E, Avila Escobedo L, Guerrero Hernández I. Renalhemodynamics and its correlation with the Child-Pugh stage in cirrhotic patients and their controls. Rev Gastroenterol Mex.2006;71:302–7.
-
Brkljaciæ B, Mrzljak V, Drinkoviæ I, Soldo D, Sabljar-MatovinoviæM, Hebrang A. Renal vascular resistance in diabetic nephropathy: Duplex Doppler US evaluation: . 1994;192:549–54.
-
de Haan MW, Kroon AA, Flobbe K, Kessels AG, Tordoir JH, vanEngelshoven JM, et al. Renovascular disease in patients withhypertension: detection with duplex ultrasound. J Hum Hypertens.2002;16:501–7
-
Keogan M, Kliewer M, Hertzberg B, DeLong DM, Tupler RH, CarrollBA. Renal resistive indexes: variability in Doppler US measurementin a healthy population. Radiology. 1996;199:165–169
-
Sigirci A , Hallac T, Akyncy A, Temel I, Gülcan H, Aslan M, et al.Renal interlobar artery parameters with duplex Dopplersonography and correlations with age, plasma renin, andaldosterone levels in healthy children. AJR Am J Roentgenol.2006;186:828–32.
|
|
|
 |
|
|