|
|
|
|
 |
 |
| |
 |
|
|
Original Articles |
|
|
|
|
|
Keywords :
hepatitis C, pegylated interferon, compensated cirrhosis; genotype 3; HCVcirrhosis |
|
|
Amna Subhan Butt, Khalid Mumtaz, Irum Aqeel, Hasnain A Shah, Saeed Hamid, Wasim Jafri
Section of Gastroenterology Department of Medicine
The Aga Khan University Hospital
Karachi, Pakistan.
Corresponding Author:
Dr. Khalid Mumtaz
Email: khalid.mumtaz@aku.edu
DOI:
http://dx.doi.org/
Abstract
Background: Chronic hepatitis C (CHC) virus infection in patients with cirrhosis is difficult totreat. There is limited data on the outcome of treatment for genotype 3 HCV infection withcirrhosis.
Aims: To determine sustained virological response (SVR) and its predictive factors in patientswith cirrhosis due to genotype 3 HCV infection treated with pegylated interferon and ribavirin(RBV).
Methods: Consecutive patients with compensated cirrhosis due to HCV genotype 3 withpositive HCV RNA treated with peg-IFN and RBV in our Gastroenterology Clinics duringNovember 2005 to December 2006 were included in this study. Cirrhosis was diagnosed onthe basis of liver biopsy and/or biochemical testing and ultrasound of abdomen. Primary endpoint of treatment was SVR.
Results: Of 66 patients, 32 (48.5%) were male. The mean age was 46.2±10.1 years; therewere 61 (92.4%) patients with Child’s A cirrhosis followed by 5 (7.6%) with Child’s B type. 33(50%) patients received pegylated interferon alfa-2a (180 µg/wk) with ribavirin and 33 (50%)received pegylated interferon alfa 2b (1 µg /kg/week) with ribavirin. EVR was achieved in 44(66.7%), and ETR in 46 (69.7%); overall SVR was achieved in 38 (57.6%) patients. Factorspredictive of SVR were age (p value = 0.03), treatment naïve status (p value = 0.04) and EVR(p value<0.001). Five patients were unable to complete the treatment due to side effects orcytopenias.
Conclusions: Treatment of patients with HCV genotype 3, compensated cirrhosis, withpegylated interferon and ribavirin is effective and well tolerated.
|
48uep6bbphidvals|170 48uep6bbphidcol4|ID 48uep6bbph|2000F98CTab_Articles|Fulltext Hepatitis C (HCV) is the second most common chronic viralinfection affecting 170 million people worldwide.[1,2,3] HCV isresponsible for 25-30% of global cases of cirrhosis associatedwith an annual risk of hepatic decompensation andhepatocellular carcinoma (HCC) in up to 5% and 1-4% cases,respectively.[1,4,5] Treatment with pegylated interferon (Peg-IFN)and ribavirin (RBV) is considered the first line treatment forhepatitis C. There is sustained virological response (SVR) in40-50% and approximately 80% in HCV genotype 1 and 2/3,respectively.[3,4] However, the response to the antiviral treatmentdepends upon many host- and virus-related factors includingage, gender, HCV genotype and viral load and stage of liverfibrosis.[6] Liver fibrosis is an important negative prognosticfactor in patients with HCV infection that makes the prognosisdismal.[7] Antiviral therapy in HCV-related cirrhosis is nowrecommended in order to stop viral replication and prevent HCC and ultimately hepatic decompensation.[3,4,8] Moreover, theachievement of an SVR is associated with a significant halt inthe process of hepatic fibrosis,[9] reduction of liver-relatedmortality, and risk of complications.[7,10,11] Nevertheless, theresponse rates to anti-viral therapy differ with the stage ofhepatic fibrosis.[12] Patients with cirrhosis have a lower chanceof clearing serum HCV-RNA and are more prone to interferonand ribavirin related adverse effects than patients withoutcirrhosis.[13] Upto 38% of SVR has been reported in subjectswith advanced fibrosis after standard interferon and RBVtherapy. On the other hand, SVR by pegylated interferonmonotherapy is reported to be 30% in patients with HCVcirrhosis.[14]
In Pakistan HCV infection reportedly affects approximately10 million people and is the most common cause of chronicliver disease.2 The HCV genotype type 3 is the most prevalent genotype affecting 67-87% cases.[15,16,17] Due to lack of medicalcoverage by the government and poor economical state, mostof our patients with genotype 3 HCV infection are treated withstandard interferon and RBV therapy.[18] HCV genotype 3 isconsidered easy to treat, however such data has beenextrapolated from subgroup analysis in larger trials, mostlyconducted on a Caucasian population with genotype otherthan 3.[6,19] In addition, In addition, available data concerning rates of SVRwith peg-IFN and ribavirin therapy in patients with genotype 3HCV-related cirrhosis are limited. Lower rates of SVR forgenotype 3 have been reported as compared to genotype 2 infew recent clinical trials after peg-IFN and RBV therapy.[20]However, many patients with advanced liver disease areusually excluded from pivotal randomised controlled studies.Hence, data regarding efficiency and tolerability of peg-IFNand RBV combination therapy for genotype 3 HCV-relatedcirrhosis is limited. There is no data available from Pakistanon treatment of patients with genotype 3 HCV-related cirrhosiswith peg-IFN and RBV.
Therefore, we conducted an analysis of HCV genotype 3cirrhotic patients treated with peg-IFN alfa 2a or 2b and RBV atthe Aga Khan University Hospital, Karachi, Pakistan. The aimof the study was to determine the sustained virologicalresponse (SVR) and factors predicting SVR in patients withcompensated cirrhosis due to HCV genotype 3 infectionstreated with peg-INF alfa 2a or 2b and ribavirin.
Patients and Methods
Study population
This was a prospective study. Consecutive patients withcompensated cirrhosis with Child Turcotte Pugh (CTP) ClassA and B due to genotype 3 HCV infection visitingGastroenterology Clinics of The Aga Khan University Hospital,Karachi, Pakistan from November 2005 to December 2006were included in the study. Cirrhosis was defined either asliver biopsy-proven cirrhosis (METAVIR stage 4)[21] or, in theabsence of liver biopsy, as an AST–platelet ratio index (APRI)score greater than two on at least two occasions in the 6months preceding treatment.[22] Features of cirrhosis onultrasound such as irregular margins of liver, reduced size ofliver, splenomegaly and/or dilated portal vein were also usedas indicators for liver cirrhosis on ultrasound abdomen. Allpatients had detectable anti-HCV antibody (by ELISA-3) andHCV RNA PCR (COBAS Amplicor HCV qualitative assay) inserum with normal or elevated ALT. Those who were non-responders or had relapsed to prior treatment with standardinterferon alfa 2a or 2b with or without RBV i.e. non-naïvepatients were also included. HCV genotyping was performedby HCV-PCR reverse hybridisation (INNOLIPA) technique. Alllaboratory tests were performed at the central laboratory ofThe Aga Khan University Hospital, Karachi.
Patients were excluded from the study who had 1) HCV-related decompensated cirrhosis; defined as ascites,portosystemic encephalopathy, hepatorenal syndrome,hepatocellular carcinoma (HCC) and recurrent variceal bleed,2) concomitant HBV, HDV or HIV infection, 3) major psychiatricillness, 4) hemoglobin < 8 g/dL, neutrophil count <1500 cells/mL , platelet count < 85,000/dL (4), 5) serum creatinine >1.5mg/dL, 6) concomitant metabolic or autoimmune liver disease,7) post liver transplant patients, 8) pregnancy, 9) uncontrolledseizures, 10) severe heart disease or other absolutecontraindications for the treatment.
All patients had received either peg-IFN alfa 2a (Pegasys;Hoffmann-La Roche, Basel, Switzerland) 180 µg/weeksubcutaneously along with RBV or peg-IFN alfa 2b (Peg-Intron;Schering-plough, Kneilworth, NJ) 1.0 µg/kg/week along withRBV. RBV was given as 10-12 mg/kg in 2 to 3 divided doses.The decision to administer peg-IFN alfa 2a or 2b was basedon the primary physician’s discretion.
Assessment of response to antiviral therapy
The therapeutic responses were assessed as follows: (i) earlyvirological response (EVR) defined as undetectable HCV RNA(<50 IU/mL) in serum after 12 weeks therapy, (ii) end oftreatment response (ETR) defined as undetectable HCV RNAin serum at the completion of treatment which was assessedat 36 and 48 weeks depending on the duration of treatment,(iii) sustained virological response (SVR), defined asundetectable HCV PCR 24 weeks after completion of antiviraltherapy (iv) non-responders (NR) defined as lack of clearanceof HCV RNA at any point during the therapy and (v) relapser(RR), defined as re-appearance of HCV RNA within 24 weeksafter completion of therapy.
Monitoring and follow-up of patients
Patients were assessed in out-patient clinics, initially 2 weeklyfor 1 month then 4 weekly until the end of treatment. Followingtreatment follow-up visits were conducted at weeks 12 and 24post-treatment. Physical signs for hepatic decompensation,adverse effects of the antiviral therapy, complete blood countand ALT were recorded on each visit. ALT and HCV PCR weretested at weeks 12, 24, end of treatment and 24 weeks aftercompleting the treatment. Treatment was terminated in caseof clinical hepatic decompensation or hemoglobin < 7.0 g/dL,platelets <50,000/mm3. However, peg-IFN or RBV dose wasmodified and erythropoietin and/or granulocyte-colonystimulating factor (G-CSF) were given in situations wherehemoglobin was <7 g/dL and absolute neutrophil count (ANC)<750/mm3. Patients with cirrhosis are usually prescribed atreatment of more than 6 months regardless of the HCVgenotype.[21,23] We offered treatment for 36 weeks to patientswho had achieved clearance of virus at 12 weeks, i.e. achievedEVR; the treatment was extended to 48 weeks if the EVR wasnot achieved but HCV PCR became undetectable at 24 week.
Clinical decompensation was defined as the developmentof ascites, hepatic hydrothorax, and porto-systemicencephalopathy or variceal bleed during treatment. The primaryend point was SVR. Secondary end points were drugtolerability (i.e. number of patients who completed the treatmentprotocol, clinical or biochemical worsening and death). Datawere also analysed to determine the predictors of SVR outcome.
The study was approved by the Ethical Review Committeeof The Aga Khan University Hospital, Karachi.
Statistical Analysis
Data were analysed using SPSS for windows version 16.0(SPSS Inc, Chicago, Illinois, USA). Results were presentedas mean ± standard deviation for quantitative variables andfrequencies (percentages) for qualitative variables. Age,gender, BMI, previous treatment status, Child’s class, baselinehemoglobin, total leukocyte count (TLC), platelet count, prothrombin time (PT), total bilirubin, albumin, alanine amino-transaminase (ALT), type of pegylated interferon used, andEVR were considered potential predicting factors for SVR. Toassess the association between SVR and categoricalvariables, the Chi-square or Fisher exact test was used, whereappropriate. Further, to assess the difference in proportionsof the SVR and non-SVR groups and quantitative variables,the independent sample t-test was used.
To evaluate potential predicting factors for SVR, univariateand multivariate logistic regression analyses were performed.Factors that were significant in the univariate analysis wereused in the multiple logistic regression models. A p value <0.05 was taken to be significant.
Results
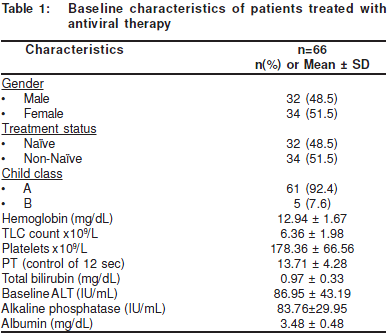
From November 2005 to December 2006, 350 patientsreceived anti-viral therapy for HCV. Out of 350, 66 (18.86%)patients fulfilled the eligibility criteria and were treated withpeg-IFN and RBV. Over all, there were 32 (48.5%) male and34 female patients; the mean age of the patients was 46.23 ±10.1 years. The mean body mass index (BMI) was 22.36 ± 3.1kg/m2. Other characteristics and baseline laboratoryparameters of patients are described in Table 1.
Out of 66 patients, 33 (50%) received injection peg-IFNalfa 2a, 180 µg/week subcutaneously along with RBV and theother 33 (50%) patients received weight-based peg-IFN alfa2b, 1 µg/kg/week along with RBV. Among those who receivedpeg-IFN alfa 2b, 2, 8, 18 and 5 patients received 50 µg, 80 µg,100 µg and 120 µg/week of doses, respectively, based ontheir body weight. All patients received RBV 10-12 mg/kg in 2–3 divided doses. Amongst the non-naïve patients the durationbetween previous standard interferon and RBV therapy andcurrent peg-IFN and RBV therapy ranged from 7-72 months.There were 46 (67.7%) patients who received 36 weekstreatment and 15 (22.7%) patients received 48 weekstreatment, as they were unable to achieve EVR at 12 weeks.
Out of 66 patients the treatment was completed by 61(92.42%) patients. Overall EVR was achieved in 44/66 (66.7%)cases while end of treatment response (ETR) was achievedby 46/66(69.7%) cases. However, among all patients SVR wasachieved in 38/66 (57.6%) patients. Moreover, SVR was achieved in 38/61 (62.3%) patients who were able to completeanti-viral therapy.
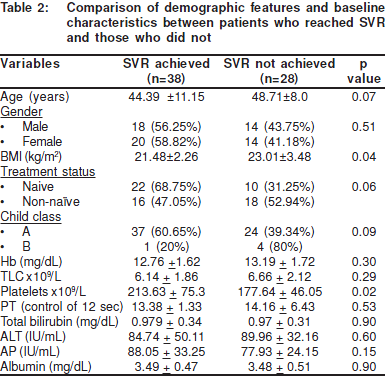
When the demographic features and baselinecharacteristics were compared between patients who had andwho had not achieved SVR there was no statistically significantdifference except in the BMI (p value = 0.04) and baselineplatelet counts (p value = 0.02) (Table 2). Baseline plateletcounts were significantly higher amongst those who achieved SVR (p value = 0.02). Although the mean BMI was within normalrange in both groups, it was significantly higher (23.01±3.48vs. 21.48±2.26, p value = 0.04) in those who were unable toachieve SVR.
Out of 34 non-naïve patients 15 (44.1%) were non-responders and 19 (55.9%) had relapsed to conventionalinterferon with or without RBV. However, after treatment withpegylated interferon and ribavirin, 6/15 (40%) non-respondersand 10/19 (52.6%) relapsers achieved SVR (p value = 0.46).Hence, similar response was observed with pegylatedinterferon and ribavirin among non-responders and relapsersfollowing initial treatment.
EVR was achieved in 34/38 (89.5%) patients who wereable to achieve SVR later on as compared to 10/28 (35.71%)patients, who did not achieve SVR (p value <0.001, OR 0.065,95% CI 0.018-0.238). Furthermore, a higher proportion ofpatients who achieved ETR had achieved SVR as comparedto those who failed to achieve SVR [38 (100%) vs. 8 (28.57%)], p value<0.001, OR 0.174, 95% CI 0.093-0.326). Therewas no difference in achieving SVR for those who receivedpeg-IFN alfa 2-a along with RBV or peg-IFN alfa 2-b and RBV(28% vs. 28.8%, p-value 0.59).
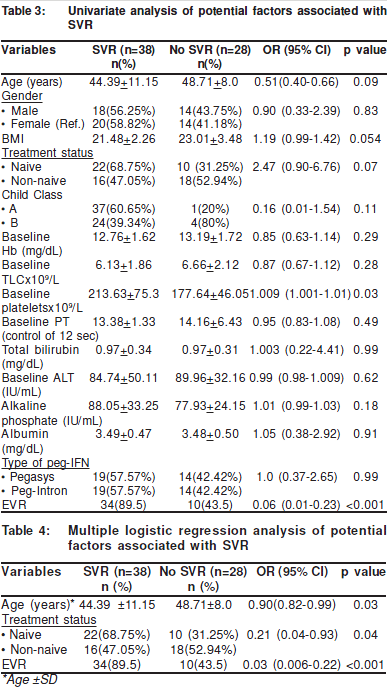
On univariate analysis younger age (p value = 0.09), lowerBMI (p value = 0.054), Child’s class A (p value = 0.11), naïve totreatment (p value = 0.07), baseline platelet count (p value =0.03), baseline alkaline phosphatase (p value = 0.18) andachievement of EVR (p value<0.001) were found to besignificant predicting factors for SVR (Table 3).
However, on multivariate logistic regression analyses age(p-value 0.03), naïve status for treatment (p-value 0.041) andEVR (p-value < 0.001) were found to be significant predictingfactors for SVR (Table 4).
Due to the development of anaemia and neutropenia onanti-viral therapy, 10 (15.15%) patients required supportiveerythropoietin or granulocyte colony stimulating factor (G-CSF)during treatment. Dose reduction of peg-IFN and RBV wasrequired in 5 (8.2%) patients due to cytopenias, markedlethargy and myalgias.
Five (7.6%) patients were unable to complete the treatmentdue to either poor tolerance or various side effects notresponding to supportive treatment. Two patients developedworsening thrombocytopenia and absolute neutropenia notresponding to G-CSF therapy. Refractory anaemia that did notrespond to erythropoietin treatment developed in two cases;one each developed ascites and hepatic encephalopathy.There were no death during the treatment period in thesecirrhotic patients.
Discussion
We conducted an analysis of 66 patients who hadcompensated cirrhosis caused by hepatitis C genotype 3.Overall EVR and ETR were achieved in 44 (66.7%) and 46(69.7%) cases, respectively. Out of 66 patients SVR was achieved in 38 (57.6%) patients including those who have notcompleted the full treatment. Moreover, SVR was achieved 38/61 (62.3%) patients who were able to complete anti-viraltherapy.
In an analysis of 28 patients with HCV-related cirrhosisfrom India,[24] ETR and SVR were reported in 24 (85%) and 15(53%) cases, respectively. However, they found a high relapserate of 38% within 6 months following completion of antiviraltherapy. Furthermore, dose modification was required in 2(7.14%) cases and the treatment had to be stopped in 3 (11%)cases. One death was reported due to worsening liver failurein this study. In comparison to this study, lower ETR wasreported in our patients but our patients were able to achievehigher SVR and there was no relapse six month after thecompletion of therapy. This difference in results by Sood et almight be explained due to inclusion of HCV genotypes otherthan type 3 in their study.24 In a recently published study fromItaly in patients with histologically proven HCV cirrhosis, peg-IFN and RBV therapy was compared with standard interferonand RBV; SVR rates were significantly higher in genotype 2/3patients than in genotype 1 patients (69% versus 25%;p<0.0001). These results are in line with ours.[25] Similarly, a randomised control study from Switzerland has reportedimproved SVR in HCV-related cirrhosis with genotypes 2 and3; the results were much better in treatment naïve patients.[14]
In another recent study conducted by Horoldt et al,[6] out of61 patients 43 (70%) patients had achieved ETR, howeveronly 39% achieved SVR; 35% genotype 1 and 39% of genotype3 were able to achieve SVR. Failure to achieve SVR was foundto be associated with lower platelet and neutrophil counts,and albumin level. Higher ETR and SVR reported in our studymay be due to younger age and female gender of patients.Moreover, in the study by Horoldt et al the overall sample sizeand subgroups of different genotypes were small; thereforeperhaps not enough to detect a difference with differentgenotypes. However, SVR of 57.6% in cirrhotic patients treatedwith peg-IFN and RBV therapy in our study is comparable toreports from Italy and Switzerland in HCV genotype 3patients.[14,25]
The efficacy of pegylated interferon alfa-2a or 2b alongwith RBV in cirrhosis has been studied in various reports.Fried et al, have found SVR in 43% cases among patients withbridging fibrosis or compensated cirrhosis treated with peg-IFN alfa 2a and RBV for 48 weeks.[26] Moreover, the efficacy of48-week therapy with peg-IFN alfa 2b 1.5 µg/kg/week plusRBV was compared against conventional IFN alfa-2b plusRBV in a randomised controlled trial. SVR was achieved in48% cases who received peg-IFN alfa-2a and RBV. Consistentwith the results of these studies no difference was found inSVR among those who were treated with peg-IFN alfa-2a oralfa-2b along with RBV (28% vs. 28.8%, p value = 0.598).
Besides genotypes other than 2 and 3, advanced age,male gender, obesity, high pretreatment viral load, degree offibrosis and previous treatment with IFN and RBV therapy arethe factors associated with worse outcome after anti-viraltherapy for HCV.[27] In our study younger age, lower BMI, child’sclass A, naïve to treatment, higher base line platelets, alkalinephosphatase and EVR were found to be significant predictingfactors for SVR on univariate analysis. However, amongstthese, only younger age, naïve to treatment and EVR were thesignificant predictors on multivariate logistic regressionanalyses. Yu et al studied the predictive value (PPV) of rapidvirological response (RVR) and EVR on SVR.[28] The positive predictive value of RVR and EVR were 86.7% (39/45) and71.9% (64/89), respectively. Nonetheless, there was nostatistically significant difference between PPV of RVR andEVR.[28] Consistent with the evidence EVR and ETR were foundas significant predicting factors for SVR in our study.
There is evidence that SVR after peg-IFN and RBV for 24 to48 weeks associated with resolution of chronic hepatitis inapproximately half of patients.[29] There has beenrecommendations to stop anti-viral therapy in the absence ofEVR.[4] However, the value of more prolonged therapy (36–48weeks) is now a major concern of clinicians andresearchers.[23,29,30] Henceforth, our 46 (67.7%) patientsreceived peg-IFN and RBV for 36 weeks and 15 (22.7%)patients received 48 weeks treatment, as they were unable toachieve EVR at 12 weeks.
Safety and tolerability of IFN and RBV are major points ofconcern in patients with cirrhosis. Bone marrow suppressionis associated with IFN therapy while RBV can lead to significanthemolysis.[31] However, our patients tolerated the antiviraltherapy well. Supportive treatment with erythropoietin and G-CSF does not affect SVR but helps continue peg-IFN and RBV;hence their use during antiviral therapy for HCV has beensupported.[21] In addition to anti-viral therapy, 10 (15.15%) ofour patients required erythropoietin or G-CSF during treatment.Dose reduction of peg-IFN and RBV was required in 5 (8.2%)patients due to cytopenias, marked lethargy and myalgias thatwas much lower than that was reported by Abergel et al (12.7-35.64% ) with different doses of peg-IFN alfa-2b plus RBV[19]and 68-78% dose reduction rate with peg-IFN alfa-2a plusstandard or reduced dose of RBV.[14] However, 2 (3%) of ourpatients developed worsening thrombocytopenia and absoluteneutropenia not responding to G-CSF therapy and 2(3%)developed refractory anemia. One (1.5%) patient developedascites and hepatic encephalopathy. No mortality was reportedduring the treatment period. Five (7.6%) patients were unableto complete the treatment due to either poor tolerance or variousside effects not responding to supportive treatment that wasagain lower then few other studies.[14,19] Henceforth, thediscontinuation rates for antiviral therapy were lower alongwith better tolerability of peg-IFN and RBV in our study patients.
However, there are a few limitations of this study. First, thestudy sample size is limited, secondly, it was a single centrestudy and a majority of them had Child’s A cirrhosis, thirdly, itwas an observational study and assignment of peg-IFN alfa2a or 2b was on the physician’s discretion; hence the studypopulation received both types of peg-IFN.
These limitations can be resolved by observing that themajority of studies in cirrhotic patients have lower numbers ofpatients particularly if a subgroup analysis is performed forgenotype 3 patients[6,14,24,25] The efficacy of two types of pegylatedinterferon in hepatitis C is reported in a Cochrane protocol.[32]According to personal communication with the author theresults are similar with the two types of pegylated interferons.Therefore type of pegylated interferon can be excluded as aconfounder in the results. Fourthly, patients naïve or non-naïveto peg-IFN and RBV were both included in the study, howeverno difference was found in SVR when subgroup analysis wasperformed to determine the type of peg-IFN used and thetreatment status. However, more prospective studies orrandomised controlled trials are needed to evaluate theefficiency and tolerability of peg-IFN and RBV therapy in patientswith compensated cirrhosis and to find out the optimal duration of therapy or additional therapy required in non-responders orrelapsers.
Conclusion
Treating patients with compensated cirrhosis due to HCVgenotype 3 infection, with pegylated interferon and ribavirin, iseffective and tolerated though supportive treatment witherythropoietin and G-CSF may be required in some cases.Our 34 (51.5%) patients were non-responders or relapsers toprior standard INF with or without RBV therapy. However,amongst the non-naïve patients 40% of non-responders and52.63% of relapsers attained SVR.
References
-
Alter MJ. Epidemiology of hepatitis C virus infection. World JGastroenterol. 2007;13:2436–41.
-
Raja NS, Janjua KA. Epidemiology of hepatitis C virus infection inPakistan. J Microbiol Immunol Infect. 2008;41:4–8.
-
Craxi A, Laffi G, Zignego AL. Hepatitis C virus (HCV) infection: asystemic disease. Mol Aspects Med. 2008;29:85–95.
-
McCaughan GW, Omata M, Amarapurkar D, Bowden S, Chow WC,Chutaputti A, et al. Asian Pacific Association for the Study of theLiver consensus statements on the diagnosis, management andtreatment of hepatitis C virus infection. J Gastroenterol Hepatol.2007;22:615–33.
-
Weigand K, Stremmel W, Encke J. Treatment of hepatitis C virusinfection. World J Gastroenterol. 2007;13:1897–905.
-
Horoldt B, Haydon G, O’Donnell K, Dudley T, Nightingale P, MutimerD. Results of combination treatment with pegylated interferon andribavirin in cirrhotic patients with hepatitis C infection. Liver Int.2006;26:650–9.
-
Zeuzem S. Treatment of chronic hepatitis C virus infection inpatients with cirrhosis. J Viral Hepat. 2000;7:327–34.
-
Omata M, Yoshida H, Shiratori Y. Prevention of hepatocellularcarcinoma and its recurrence in chronic hepatitis C patients byinterferon therapy. Clin Gastroenterol Hepatol.2005;3:S141–3
-
Poynard T, McHutchison J, Manns M, Trepo C, Lindsay K, GoodmanZ, et al. Impact of pegylated interferon alfa-2b and ribavirin onliver fibrosis in patients with chronic hepatitis C. Gastroenterology.2002;122:1303–13.
-
Bruno S, Stroffolini T, Colombo M, Bollani S, Benvegnu L, MazzellaG, et al. Sustained virological response to interferon-alpha isassociated with improved outcome in HCV-related cirrhosis: aretrospective study. Hepatology. 2007;45:579–87.
-
Veldt BJ, Heathcote EJ, Wedemeyer H, Reichen J, Hofmann WP,Zeuzem S, et al. Sustained virologic response and clinicaloutcomes in patients with chronic hepatitis C and advancedfibrosis. Ann Intern Med. 2007;147:677–84.
-
Piccinino F, Coppola N. Antiviral treatment of HCV-related cirrhosis. Dig Liver Dis. 2007;39 Suppl 1:S96–101.
-
Fried MW. Side effects of therapy of hepatitis C and theirmanagement. Hepatology 2002;36:S237–44.
-
Helbling B, Jochum W, Stamenic I, Knopfli M, Cerny A, Borovicka J,et al. HCV-related advanced fibrosis/cirrhosis: randomizedcontrolled trial of pegylated interferon alpha-2a and ribavirin. J Viral Hepat. 2006;13:762–9.
-
Shah HA, Jafri W, Malik I, Prescott L, Simmonds P. Hepatitis C virus(HCV) genotypes and chronic liver disease in Pakistan. J Gastroenterol Hepatol. 1997;12:758–61.
-
Idrees M, Riazuddin S. Frequency distribution of hepatitis C virusgenotypes in different geographical regions of Pakistan and theirpossible routes of transmission. BMC Infect Dis. 2008;8:69.
-
Mumtaz K, Hamid SS, Moatter T, Abid S, Shah HA, Jafri W.Distribution of hepatitis C virus genotypes and its response totreatment in Pakistani patients. Saudi Medical J. 2008;29:1671–3.
-
Zuberi BF, Zuberi FF, Memon SA, Qureshi MH, Ali SZ, Afsar S.Sustained virological response based on rapid virological responsein genotype-3 chronic hepatitis C treated with standard interferonin the Pakistani population. World J Gastroenterol.2008;14:2218–21
-
Abergel A, Hezode C, Leroy V, Barange K, Bronowicki JP, Tran A,et al. Peginterferon alpha-2b plus ribavirin for treatment of chronichepatitis C with severe fibrosis: a multicentre randomizedcontrolled trial comparing two doses of peginterferon alpha-2b. J Viral Hepat. 2006;13:811–20.
-
Aghemo A, Rumi MG, Soffredini R, D’Ambrosio R, Ronchi G, DelNinno E, et al. Impaired response to interferon-alpha2b plus ribavirinin cirrhotic patients with genotype 3a hepatitis C virus infection. Antivir Ther. 2006;11:797–802.
-
Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis,management, and treatment of hepatitis C: an update. Hepatology.2009;49:1335–74.
-
Shaheen AA, Myers RP. Diagnostic accuracy of the aspartateaminotransferase-to-platelet ratio index for the prediction ofhepatitis C-related fibrosis: a systematic review. Hepatology.2007;46:912–21.
-
Farrell GC. New hepatitis C guidelines for the Asia-Pacific region:APASL consensus statements on the diagnosis, management andtreatment of hepatitis C virus infection. J Gastroenterol Hepatol.2007;22:607–10.
-
Sood A, Midha V, Sood N, Bansal M. Pegylated interferon alfa 2band oral ribavirin in patients with HCV-related cirrhosis. Indian J Gastroenterol. 2006;25:283–5.
-
Roffi L, Colloredo G, Pioltelli P, Bellati G, Pozzpi M, Parravicini P, etal. Pegylated interferon-alpha2b plus ribavirin: an efficacious and well-tolerated treatment regimen for patients with hepatitis C virusrelated histologically proven cirrhosis. Antivir Ther.2008;13:663–73.
-
Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, GoncalesFL Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitisC virus infection. N Engl J Med. 2002;347:975–82.
-
Kemmer N, Neff GW. Managing chronic hepatitis C in the difficult-to-treat patient. Liver Int. 2007;27:1297–310.
-
Yu JW, Wang GQ, Sun LJ, Li XG, Li SC. Predictive value of rapidvirological response and early virological response on sustainedvirological response in HCV patients treated with pegylatedinterferon alpha-2a and ribavirin. J Gastroenterol Hepatol.2007;22:832–6.
-
Di Bisceglie AM, Shiffman ML, Everson GT, Lindsay KL, EverhartJE, Wright EC, et al. Prolonged therapy of advanced chronic hepatitisC with low-dose peginterferon. N Engl J Med.2008;359:2429–41.
-
Ide T, Hino T, Ogata K, Miyajima I, Kuwahara R, Kuhara K, et al. ARandomized Study of Extended Treatment With Peginterferon alpha-2b Plus Ribavirin Based on Time to HCV RNA Negative-Status inPatients With Genotype 1b Chronic Hepatitis C. Am J Gastroenterol.2009;104:70–5
-
Iacobellis A, Ippolito A, Andriulli A. Antiviral therapy in hepatitis Cvirus cirrhotic patients in compensated and decompensatedcondition. World J Gastroenterol. 2008;14:6467–72.
-
Tahany A KT, Goran H, Mahasen M, Davor S, Christian G. Pegylatedinterferon alpha 2a versus pegylated interferon alpha 2b forchronic hepatitis C. Cochrane Database of Systematic Reviews.[Cochrane review]. 2009(1).
|
|
|
 |
|
|