N.R. Sawalakhe, S.Nistala, M. Sasidharan, R.T.Narendaran, A.D.Amrapurkar, R.M.Joshi, P.M. Rathi
Department of Gastroenterology
B.Y.L.Nair Charitable Hospital and T.N.M.C Mumbai Central,
Mumbai - 400008 Maharashtra,
India.
Corresponding Author:
Dr.Pravin M. Rathi
Email:rathipm@liver.com
DOI:
http://dx.doi.org/
48uep6bbphidvals|250 48uep6bbphidcol4|ID 48uep6bbph|2000F98CTab_Articles|Fulltext Gastric carcinoid accounts for less than 1% of gastric neoplasms and 11-41 % of all carcinoid tumors.[1] They are classified into three types depending on clinical and histological characteristics. Type I is associated with hypergastrinemia, achlorhydria, enterochromaffin like (ECL) cells hyperplasia , and chronic atrophic gastritis type- A (CAGA), with or without pernicious anaemia.1 Type II is associated with Zollinger-Ellison syndrome(ZES) and multiple endocrine neoplasia (MEN-1) syndrome.1 Type III tumors are sporadic, large, solitary and invasive tumors.1 Gastric carcinoid tumors are mostly multifocal (usually type I and type II); solitary tumors are rare [2]. They are usually asymptomatic or present with vague upper abdominal symptoms. Occasionally, they may present with unexplained weight loss, gastrointestinal bleeding, or anaemia which at times could be severe.[2]
Case Report
A 60 year old, female presented with recurrent epigastric pain for past 3 months with two to three episodes of malena, and generalized weakness. One year ago, she received blood transfusion for hematemesis without any endoscopic evaluation. Physical examination revealed severe pallor and platynychia. Vitals signs were normal. Laboratory findings showed microcytic hypochromic anaemia with hemoglobin 62g/L, hematocrit 19.4%, MCV 68fL, MCH 22pg, MCHC 26.2 g/dl, RDW 34%, platelets 450x103 /mm3, reticulocyte count 3%, serum iron 40µg/dL, total iron binding capacity (TIBC) 452µg/dL, and serum ferritin 8 ng /ml. Stool for occult blood was strongly positive. Rest of the routine laboratory tests were normal. Ultrasound of the abdomen revealed a polypoidal mass in the gastric body along the greater curvature with moderate vascularity.
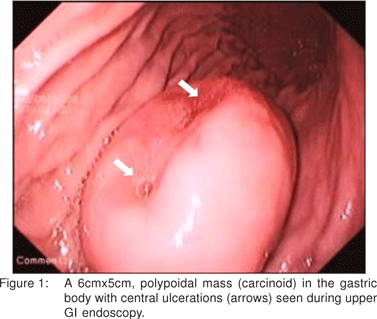
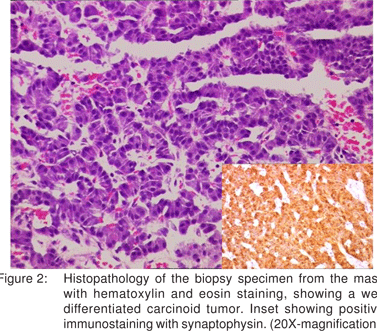
Upper GI endoscopy showed a large polyp (6cmx5cm) in the gastric body along the greater curvature with central ulcerations (Figure 1). Histopathology of the biopsies from the mass showed a well differentiated gastric neuroendocrine tumor, which stained positive for synaptophysin (Figure 2). There was no evidence of atrophic gastritis or H.pylori. Fasting serum gastrin and 24-hour urinary 5-hydroxyindoleacetic acid levels were normal [98pg/L and 3.19(2-6mg/24hr) respectively]. CT abdomen showed no evidence of lymphadenopathy or other organ metastasis. Patient also did not have any symptom of carcinoid syndrome. A diagnosis of large solitary type III gastric carcinoid was made and patient was subjected to surgery.
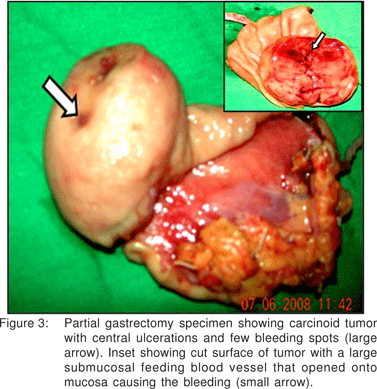
A partial gastrectomy was done at which a sessile, submucosal tumor with ulcerations and bleeding points on the mucosal surface was removed. Resected margins were free of tumor cells. Few abnormal submucosal vessels were seen on cut section that opened onto the mucosal surface and probably were the source of bleeding (Figure 3).Patient is under regular follow up for the past six months without recurrence of tumor or metastasis. Vigilant semi-annual follow-up with endoscopy and CT scan are now planned.
Discussion
Type I and type II gastric carcinoid tumors are characteristically multiple and small, with low malignant potential, occurring in the setting of hypergastrinemia which causes ECL cells hyperplasia. They develop through a sequence of “hyperplasia-dysplasia-neoplasia”. ECL-cell hyperplasia and dysplasia are identified as the precancerous lesions of ECL cell carcioid.[3] In type I tumors, chronic atrophic gastritis (CAG) [either autoimmune or due to H.pylori] causes parietal cells destruction in the fundus and body, causing achlorhydria.[4] Achlorhydria results in hypergastrinemia due to uninterrupted stimulation of antral G-cells.[4] These tumors are often multifocal, less than 2 cm in diameter found in the fundus or body . Nodal and hepatic metastasis occurs in 2%.[1,2,5] Endoscopic polypectomy is curative for tumor smaller than 1 cm.[5,6] For multiple tumors larger than 1 cm, and with abdominal symptoms, antrectomy or subtotal gastrectomy (including the antrum) is recommended.[5,6]
Type II tumors are the least common type of gastric carcinoids (5-10%). Here the hypergastrinemia is due to ZES, associated with MEN-1syndrome.[1,2,6] They are rarely seen in sporadic ZES without MEN-I. They are multicentric, variable in size and are prone for local nodal metastasis. However, tumor-related death or carcinoid syndrome is rare. Various treatment modalities have been used including endoscopic polypectomy, partial or total gastrectomy, or medical management with somatostatin analogues.[7]
Type III carcinoids are sporadic, large and solitary tumors without hypergastrinemia or CAG and represents 13% of total gastric carcinoid tumors.[1,2] 80% patients are males.[1] These tumors have overexpression of the mutated p53 protein.[8] Metastasis occurs in 66% of patients, if tumor is larger than 3cm, whereas metastasis is detected in only 10% of patients when tumor is solitary and smaller than 1 cm.[2,5] Liver metastasis causes carcinoid syndrome. These tumors have poor prognosis with 5- year survival rate of around 20%.[1,7] They require radical or extended radical gastrectomy with loco- regional lymphadenectomy, especially when there are no liver secondaries or distant metastasis.[2,7] In one series, the 5-year survival rate was 75% after extended radical gastrectomy of locally advanced tumors.[9] Simple polypectomy has a risk of recurrence and progression of the disease. Liver metastasis can be treated with multidisciplinary approach.[10] Single lesion can be resected. Various combined modalities including treatment with somatostatin analogues, hepatic artery embolisation or chemoembolisation, surgical debulking, adjuvant receptor targeted therapy and even orthotopic liver transplantation have been used in metastatic settings[10]. This patient, who had no evidence of lymphadenopathy or organ metastasis, was treated with partial gastrectomy. Although there is a theoretical risk of recurrence and dissemination, at present this patient is disease free after six months of follow up. She is still under vigilant follow up.
Review of the literature has revealed previously, very few cases of type III gastric carcinoid with significant upper gastrointestinal bleeding. Three cases had an abnormal submucosal vessel opening up onto the mucosal surface.[11,12] The cause of bleeding in our case appears to be the same. It is imperative to consider carcinoid in the differential diagnosis of a gastric polyp. Gastric carcinoids can present with upper gastrointestinal bleed leading to anaemia. The recognition of these rare tumors is important, as certain types have a high malignant potential.
References
1. Rindi G, Luinetti O, Cornaggia M, Capella C, Solcia E. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology. 1993;104:994–1006.
2. Binstock AJ, Johnson CD, Stephens DH, Lloyd RV, Fletcher JG. Carcinoid tumors of the stomach: a clinical and radiographic study. AJR Am J Roentgenol. 2001;176:947–51.
3. Solcia E, Fiocca R, Villani L, Luinetti O, Capella C. Hyperplastic, dysplastic, and neoplastic enterochromaffin- like-cell proliferations of the gastric mucosa. Classification and histogenesis. Am J Surg Pathol. 1995;19 Suppl 1:S1–S7.
4. Berger MW, Stephens DH. Gastic carcinoid tumors associated with chronic hypergastrinemia in a patient with Zollinger-Ellison syndrome. Radiology. 1996;20:371–3.
5. Jordan PH Jr, Barroso A, Sweeney J. Gastric carcinoids in patients with hypergastrinemia. J Am Coll Surg. 2004;199:552–5.
6. Kulke MH. Neuroendocrine tumors: clinical presentation and management of localized disease. Cancer Treat Rev. 2003;29:363–70.
7. Cadiot G, Cattan D, Mignon M. Diagnosis and treatment of ECL cell tumors. Yale J Biol Med. 1998;71:311–23.
8. Peny MO, Donckier V, Gelin M, Haot J, Noel JC. Sporadic carcinoid of the stomach: a highly proliferative disease with a probable role for p53 protein dysregulation. Eur J Gastroenterol Hepatol. 1999;1:677–9.
9. Schindl M, Kaserer K, Niederle B. Treatment of gastric neuroendocrine tumors: the necessity of a type-adapted treatment. Arch Surg. 2001;136:49–54.
10. Caplin ME, Hodgson HJ, Dhillon AP, Begent R, Buscombe J, DickR, et al. Multimodality treatment for gastric carcinoid tumor with liver metastases. Am J Gastroenterol. 1998;93:1945–8.
11. Dallal H J, Ravindran R, King P M, Phull P S. Gastric carcinoid tumor as a cause of severe upper gastrointestinal haemorrhage. Endoscopy. 2003;35:716.
12. Devarbhayi H, Alvares JF. Polypoid gastric carcinoid tumor presenting as hematemesis with prolapse into the duodenum. Gastrointest Endosc. 2003;57:618–20.
|