|
|
|
|
 |
 |
| |
 |
|
|
Surgical Gastroenterology |
|
|
|
|
|
Keywords :
|
|
|
Rohin Mittal, Benjamin Perakath, Suchita Chase, Mark Ranjan Jesudason, Sukria Nayak
Department of Surgery Unit 5 (Colorectal surgery)
Christian Medical College and Hospital,
Vellore, Tamil Nadu, India
Corresponding Author:
Dr. Rohin Mittal
Email: rohinmittal@gmail.com
DOI:
http://dx.doi.org/
Abstract
Background: Transanal excision is commonly used to treat lesions of the anorectum. It avoids the morbidity of radical pelvic surgery, while allowing for complete histopathological examination of the lesion.
Aim: The aim of this study was to look at the spectrum of disease treated by transanal excision, and their outcomes, in a tertiary care institute.
Methods: Records of patients who underwent transanal excision between 2004 and 2008 were reviewed. Patients were divided into three groups. 1) Resection for benign disease 2) Curative and 3) Palliative resection for malignant disease.
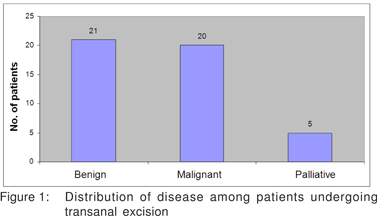
Results: Forty six patients underwent transanal excision, 21 for benign and 25 for malignant disease, 20 with curative and 5 with palliative intent. Tubulovillous adenomas and hyperplastic polyps were the commonest benign lesions. The mean follow up was 18.6 months (4-49). There was one recurrence and one patient returned with liver metastasis. Seventeen patients with adenocarcinoma, two with melanoma and one with verrucous carcinoma underwent curative resection. Three required a second local excision and two abdominoperineal excision. Mean follow up was 28 months (4-63). There were three recurrences, one requiring a local excision and two abdominoperineal excision. Four patients with malignant melanoma and one with adenocarcinoma underwent palliative resection. All these patients had good symptom palliation.
Conclusion: Transanal excision, when technically feasible, remains the treatment of choice for benign disease of the rectum. It offers good palliation of local symptoms in advanced malignant disease. It can be used in a carefully selected group of patients with early rectal cancer.
|
48uep6bbphidvals|223 48uep6bbphidcol2|ID 48uep6bbph|2000F98CTab_Articles|Fulltext Surgery of the anorectum is complicated by the presence of the anal sphincters and the need to preserve fecal continence. The treatment of choice for low rectal tumours is radical resection in the form of an abdominoperineal excision (APE). This, apart from a permanent colostomy, involves major pelvic surgery with attendant morbidity and mortality, including bladder and sexual dysfunction.
Transanal excision can be used for the treatment of benign and a few carefully selected malignant anorectal tumours[1]. It avoids a permanent colostomy and the morbidity of major pelvic surgery, at the same time not compromising outcomes.
The aim of this study was to look at the spectrum of disease treated by transanal excision and their outcomes, in a tertiary care institute.
Methods
All patients who underwent transanal excision in the unit of colorectal surgery from 2004 to 2008 were included in the study. Patients were divided into three groups.
• Group 1 - Resection for benign disease
• Group 2 - Curative resection for malignant disease
• Group 3 - Palliative resection for malignant disease
Data was collected from hospital records with special emphasis on diagnosis, histopathology, margin status including deep margin, need for re-excision or additional surgery and recurrence. Follow up information was obtained from hospital records and also by telephonic conversation.
All patients underwent preoperative imaging in the form of CT scan, MRI or endoanal ultrasound to look for local extent of disease and distant spread. The specimens were labeled and oriented in terms of margin, including deep argin, for histopathology.
Results
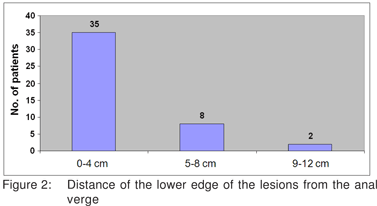
Forty six patients underwent transanal excision. There were 21 resections for benign disease and 25 for malignant disease, 20 with curative and 5 with palliative intent (Figure1). There were 36 males and 10 females. Mean age was 53.9 years (17 to 85 years). The average distance from the anal verge was 3.3 cm. Thirty five patients had lesions within 4 cm from the anal verge, 8 patients from 4 to 8 cm and 2 patients beyond 8 cm (Figure 2).
Group 1 - Benign disease (n=21)
Anorectal polyps (19/21) were the commonest benign lesions. There were 10 tubulovillous adenomas (TVA) (5 with high grade and 5 with low grade dysplasia), 4 inflammatory polyps, 2 hyperplastic polyps and 1 juvenile, serrated and fibroepithelial polyp each. Two other lesions were reported as haemorrhoidal tissue and benign rectal gastrointestinal stromal tumour (GIST).
There were 2 patients who had margin positive for dysplasia. One recurred at 4 months and 1 was asymptomatic at 7 months. Immediate re-resection was not performed in any patient.
Group 2 - Excision with curative intent (n=20)
Seventeen patients with adenocarcinoma, 2 with melanoma and 1 with verrucous carcinoma underwent curative esection. Among the adenocarcinoma, 4 were Tis, 6 were T1, 5 were T2 and 2 were Tx. (Figure 3).
Among the four patients with carcinoma in situ (Tis) , one had a positive margin and underwent reexcision. He was well on follow up at 31 months.
In patients with T1 lesions, two had a positive margin. One was advised an abdominoperineal excision based on poor prognostic factors on histopathology, and one underwent a repeat transanal excision. Average follow up in this group was 26.2 months (12-56). There was one recurrence at 9 months. This patient underwent a repeat transanal excision and was disease free at 19 month follow up.
There were 5 patients with T2 lesions. Four were suspected to have a T1 stage by preoperative workup, but were found to have a T2 lesion on histopathology. One of these underwent an APE, and three were kept on surveillance as they were not willing for APE. One of them was margin positive and underwent a wider local excision before being kept on surveillance. Two of these had a recurrence at 32 and 36 weeks and required neoadjuvant treatment followed by APE. The third was disease free at 25 months. There was one patient with a T2 lesion that underwent a local excision due to other co-morbid conditions precluding a major resection.
.gif)
Two patients (Tx) had undergone a local resection of rectal polyp elsewhere and diagnosed to have malignancy. They underwent a repeat transanal excision to ensure a negative margin.
Group 3 - Excision with palliative intent (n=5)
Four patients with malignant melanoma and one with adenocarcinoma underwent palliative resection. All these patients had distant metastasis at presentation.
All of them had good symptom palliation, but went on to have progression of disease. Two died within 1 year of the procedure from distant disease. Two had local recurrence, one underwent repeat transanal excision and one was offered APE for palliation.
Complications
1 patient had rectal perforation necessitating laparotomy and colostomy. There was no other major morbidity associated with the procedures.
Discussion
Since its introduction by Parks[2], transanal excision has been an alternative to other radical procedures for lesions of the anorectum. Its advantages include, apart from the avoidance of a permanent colostomy, shorter operating time, decreased postoperative pain, early discharge from hospital and lower morbidity and mortality[1].
Transanal excision is used for both benign and malignant disease situated in the lower or mid rectum1 and the anal canal. The excision may be full thickness or partial thickness, depending upon the depth of invasion of the lesion[3,4].
The ease of excision decreases with increasing distance from the anal verge. The transanal approach is most frequently adopted for lesions within 6-8 cm from the anal verge.[3,5] Ninety six percent (44/46) of the lesions in our patients were within 8 cm from the anal verge. For more distant lesions, other forms of local excision like Transanal Endoscopic Microsurgery (TEM)[3,6] or an anterior resection should be considered.
Although conventionally used for small tumours, even large and circumferential tumours (mostly rectal villous adenomas) can be treated transanally[4,7]. Exceptions include tumours involving a large area of the rectal mucosa or circumferential rectal lesions extending for a considerable length. In these cases, proctectomy (anterior resection or a colo-anal anastomosis with mucosectomy) may be needed[8,9]. Abdominoperineal resection is reserved for patients with invasive adenocarcinoma, in which a lesser procedure wound be oncologically incorrect[9].
Benign lesions
Any benign disease situated in the lower and mid rectum should be considered for transanal excision[1,3]. Transanal excision may also be used when the definitive diagnosis of a lesion is in doubt or unclear. It provides for complete histopathological diagnosis with a relatively simple procedure[7].
The importance of regular follow up and surveillance in these patients cannot be overstated. All patients must be followed up at regular intervals for disease recurrence as well as development of new lesions[7]. This should be even more aggressive if the margin is positive for dysplasia or the lesion has any high risk features. In our series there were 2 recurrences. One was picked up at 4 months on regular surveillance, and underwent repeat transanal excision. One patient defaulted and presented late with malignant transformation and distant metastasis.
Malignant lesions
Transanal excision should be considered for a select group of patients with anorectal adenocarcinoma. These include early lesions (Tis and T1) with no involvement of local lymph nodes, no distant disease (confirmed by imaging such as transrectal ultrasound, CT scan or MRI) and no high risk features1,3,5. In these cases the risk of local lymph node spread is considered to be between 6-13%[1,5,10], and these are considered cured after local excision. Our local recurrence rate for Tis and T1 lesions was 10% (1/10) and this was managed with a repeat local excision. There have been concerns about an increased risk of local recurrence[11,12 ,13], and these patients need to be on a regular follow up.
Lesions with high risk features such as poor differentiation, perineural invasion and lymphovascular invasion are not suited for local excision[5]. Whenever such features are detected after local excision, they should be followed by an abdominoperineal excision or anterior resection[1,3] as was done in 1 patient in our study. Local excision followed by radical surgery has not shown to compromise outcomes when compared to primary radical surgery[14].
In case of a positive margin, in the absence of any high risk features, a second local excision can be undertaken to achieve a negative margin, as was done in 3 patients in our series.
Local excision for T2 lesions is controversial. The risk of local lymph node metastasis in this group is between 17 – 22%[5 ]with a high local recurrence rate of up to 47%[15] (40% in our series). The addition of adjuvant chemo radiation has been shown to reduce the local recurrence to up to 27%.[5,16,17] Local excision of T2 lesions should therefore be reserved for patients with significant comorbidity precluding radical surgery or those not accepting a stoma[5,16] (4 out of 5 in out group). In such cases, this should be followed up with adjuvant chemoradiation.
Transanal excision can also be used in a select group of other anorectal malignancies like melanoma or verrucous carcinoma. It is important to make sure that the disease is localized to the anorectum before undertaking curative local excision.
Palliative resection
Local excision, if technically feasible, offers excellent palliation in symptomatic metastatic malignancy of the anorectum[1]. It palliates both intestinal obstruction as well as local symptoms such as bleeding, while avoiding a colostomy. Total removal of the tumour is not necessary and debulking offers good palliation of symptoms. Repeat local excision in case of recurrence may be attempted if feasible, as was done in 2 patients in our group.
Complications
Transanal excision is a relatively safe procedure with a low complication rate3,18. Minor complications include urinary retention18 and bleeding per rectum (5-10%)3,4. Major complications such as rectal perforation are rare.(1.7- %)3,4,18.
Conclusion
Transanal excision is the treatment of choice for benign lesions of the anorectum. It can be used in a carefully selected group of patients with early rectal cancer. When technically feasible, it offers good palliation for people with advanced anorectal malignancy.
Refrences
1. Piccinini EE, Ugolini G, Rosati G, Conti A. Transanal local resection for benign and malignant rectal tumours. Int J Colorectal Dis 1995;10:112–6.
2. Parks AG. Benign tumors of the rectum. In: Rob C, Smith R, Morgan CN, editors. Abdomen, rectum and anus. Clinical surgery. London, England: Butterworths; 1966. p. 541.
3. Fucini C, Segre D, Trompetto M. Local excision of rectal polyp: indications and techniques. Tech Coloproctol. 2004;8:s300–4.
4. Featherstone JM, Grabham JA, Fozard JB. Per-anal excision of large, rectal, villous adenomas. Dis Colon Rectum.2004;47:86–9.
5. Moore HG, Guillem JG. Local therapy for rectal cancer. Surg Clin North Am. 2002;82:967–81.
6. Middleton PF, Sutherland LM, Maddern GJ. Transanal endoscopic microsurgery: a systematic review. Dis Colon Rectum. 2005;48:270–84.
7. Pigot F, Bouchard D, Mortaji M, Castinel A, Juguet F, Chaume JC, et al. Local excision of large rectal villous adenomas: long-term results. Dis Colon Rectum. 2003;46:1345–50.
8. Cho SD, Herzig DO, Douthit MA, Deveney KE. Treatment strategies and outcomes for rectal villous adenoma from a single-center experience. Arch Surg. 2008;143:866–70; discussion 71-2.
9. Whitlow CB, Beck DE, Gathright JB. Surgical excision of large rectal villous adenomas. Surg Oncol Clin N Am. 1996;5:723–34.
10. Nascimbeni R, Burgart LJ, Nivatvongs S, Larson DR. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum. 2002;45:200–6.
11. Madbouly KM, Remzi FH, Erkek BA, Senagore AJ, Baeslach CM, Khandwala F, et al. Recurrence after transanal excision of T1 rectal cancer: should we be concerned? Dis Colon Rectum. 2005;48:711–9; discussion 9-21.
12. Endreseth BH, Myrvold HE, Romundstad P, Hestvik UE, Bjerkeset T, Wibe A. Transanal excision vs. major surgery for T1 rectal cancer. Dis Colon Rectum. 2005;48:1380–8.
13. Nascimbeni R, Nivatvongs S, Larson DR, Burgart LJ. Long-term survival after local excision for T1 carcinoma of the rectum. Dis Colon Rectum. 2004;47:1773–9.
14. Hahnloser D, Wolff BG, Larson DW, Ping J, Nivatvongs S. Immediate radical resection after local excision of rectal cancer: an oncologic compromise? Dis Colon Rectum. 2005;48:429–37.
15. Mellgren A, Sirivongs P, Rothenberger DA, Madoff RD, Garcia- Aguilar J. Is local excision adequate therapy for early rectal cancer? Dis Colon Rectum. 2000;43:1064–71; discussion 71-4.
16. Fortunato L, Ahmad NR, Yeung RS, Coia LR, Eisenberg BL, Sigurdson ER, et al. Long-term follow-up of local excision and radiation therapy for invasive rectal cancer. Dis Colon Rectum. 1995;38:1193–9.
17. Wagman R, Minsky BD, Cohen AM, Saltz L, Paty PB, Guillem JG. Conservative management of rectal cancer with local excision and postoperative adjuvant therapy. Int J Radiat Oncol Biol Phys. 1999;44:841–6.
18. Winburn GB. Surgical resection of villous adenomas of the rectum. Am Surg. 1998;64:1170–3.
|
|
|
 |
|
|