|
|
|
|
 |
 |
| |
 |
|
|
Quarterly Reviews |
|
|
|
|
|
Keywords :
Hepatic Osteodystrophy, Chronic liver disease |
|
|
Varun Goel, Premashis Kar
Department of Medicine
Maulana Azad Medical College
B.L.Taneja Block,
Nerw Delhi-India
Corresponding Author:
Dr Premashis Kar
Email:premashishkar@gmail.com
DOI:
http://dx.doi.org/
Abstract
Hepatic Osteodystrophy (HO) is a generic definition for the metabolic bone disease that may occur in individuals with chronic liver disease. Hepatic Osteodystrophy is an important but frequently overlooked complication, seen in chronic liver disease patients. This review article illustrates its significance, various causes and methods to diagnose this complication and recent advances and recommendations to treat Hepatic Osteodystrophy. Two distinct bone metabolic processes, osteoporosis (OP) and osteomalacia (
OM ) are combined together in various proportions in HO syndromes. It has been described in association with most types of chronic liver disease both cholestatic and non-cholestatic. Primary biliary cirrhosis (PBC) is the condition causing osteopenia more frequently, but other cholestatic liver diseases like primary sclerosing cholangitis (PSC), haemochromatosis and alcoholic liver disease are also frequently associated with this disorder. The pathogenesis of bone disease in both adults and children with chronic cholestasis is not completely understood. There has been considerable disagreement regarding the relative importance of osteomalacia versus osteoporosis as the factors leading to osteopenia of liver disease. It can significantly affect morbidity, and quality of life of these patients. Fractures are also associated with an excess mortality. Bone mineral density measurement is the best way to assess the presence and severity of osteopenia in CLD patients, while laboratory tests give important information about the metabolic status of the bone. Since advanced HO is difficult to treat and adversely affects both the quality of life and the long-term prognosis of patients with chronic liver disease, special care is required in order to prevent the development of clinical bone disease in individuals with advanced hepatic disease.
Conclusion: Hepatic Osteodystrophy is under-recognized and less attended complication of CLD. Multiple factors contribute to the development of hepatic Osteodystrophy. Newer diagnostic modalities have improved the detection of HO and Vitamin D repletion, calcium supplementation and Bisphosphonates seem promising. The best course of management for these patients is to review the individual risk factors for osteoporosis, obtain a bone mass measurement, and prescribe age and disease-specific therapies.
|
48uep6bbphidcol4|ID 48uep6bbphidvals|303 48uep6bbph|2000F98CTab_Articles|Fulltext Hepatic osteodystrophy (HO) was first described in 1960; this term was used for metabolic bone disease seen in chronic liver disease patients. Prior it was thought that metabolic bone disease (MBD) is seen in patients with cholestatic liver disease only, but recent research suggests that it is prevalent in other chronic liver diseases (CLD) also. It is an important complication of CLD[1] since it can result in fragility fractures,[2] which have a significant impact on morbidity, quality of life and even survival.
MBD is directly proportional to duration and severity of CLD and with the improvement in survival of patients with chronic liver disease[3,4] and development of liver transplantation, the clinical significance of hepatic osteodystrophy has increased. Moreover, availability of sensitive bone markers and DEXA (dual energy X-ray absorptiometry) scan has shown increased prevalence of HO. Hepatic osteodystrophy is of two types: 1. Osteoporosis which is similar to postmenopausal and aging-related bone loss; this type is more frequently seen and trabecular (cancellous) bone is more severely affected than cortical bone 2. osteomalacia which is found in cases of advanced liver disease and malabsorption.
Causes
· Chronic liver diseases: - Alcoholic liver disease, autoimmune hepatitis, chronic viral hepatitis, primary biliary cirrhosis, primary sclerosing cholangitis, liver failure and liver transplantation
· Malabsorption: - Protein-calorie malnutrition, calcium, magnesium, Vitamin B12, Vitamin. K and Vitamin D deficiencies.
· Nutritional disorder of excess: - Alcohol and phosphorus excess.
· Medical interventions: - Glucocorticoids use >3 months or multiple courses (prednisolone > 5 mg/day), loop diuretics, cholestyramine, immunosuppressive agents.
Prevalence
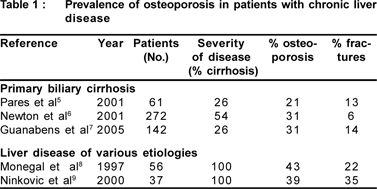
Prevalence of osteoporosis in patients with CLD is 20-60% which partly depends on patient selection and diagnostic criteria. Actually, chronic liver disease consists of cirrhotic and non-cirrhotic patients, and analyses have generally been performed not only in cirrhotics with a broad range of disease severity, but also in pre-cirrhotic patients. The prevalence of fractures in patients with liver disease ranges from 6% to 35%. (Table 1)
Bone remodeling
Maintenance of skeletal integrity involves a sequential coupling of osteoclast-induced bone resorption with osteoblast mediated bone formation and subsequent osteoid mineralization at remodeling sites in combination termed as basic multicellular units (BMU). Various factors which affect functioning of BMU are PTH, active Vitamin D, calcium, phosphate, IGF-1, TGF-_ and gonadal steroids. The major controversy regarding the mechanism of osteoporosis in CLD is whether it is because of less bone formation or more bone resorption. In low turnover osteoporosis (80%), it is assumed that bone remodeling unit (BMU) activity is severely affected and it is seen in parenchymal liver disease, whereas in high turnover osteoporosis (20%) BMU activity is increased (osteoclast) and it is seen in cholestatic liver diseases.
Pathogenesis: multifactorial
Various potential inciting factors that either directly or indirectly alter bone mass are IGF-1 deficiency, hyperbilirubinemia, hypogonadism, alcohol, subnormal 25- hydroxyvitamin D levels, vitamin D receptor genotypes, vitamin K, osteoprotegerin (OPG) and receptor activator of nuclear factor Kb ligand (RANKL) interactions and concurrent use of drugs like cholestryramine, frusemide, glucocorticoids and immunosuppressive agents. IGF-1 is synthesized in the liver. It is a bone collagen and osteoblast stimulator. In a rat model of hepatic osteodystrophy, low-dose IGF-1 increased bone mass and bone density. Lower serum IGF-1 levels were seen in CLD patients.[10]
Increased bilirubin levels found in CLD patients results in decrease IGF-1 generation and have inhibitory effect on osteoblast. However Smith et al concluded that hyperbilirubinemia is not a major contributing factor to altered bone mineral density in patients with chronic liver disease.[11] Hypogonadism is an established risk factor for osteoporosis and chronic liver disease accelerates the development of hypogonadism due to (1) reduced hypothalamic release of gonadotrophins and (2) primary gonadal failure. In CLD patients subnormal serum concentrations of vitamin D are not a consequence of reduced hepatic hydroxylation, but due to malabsorption, increased urinary excretion, and reduced enterohepatic circulation of vitamin D.
Although vitamin D deficiency per se is likely not implicated in the development of hepatic osteodystrophy, reduced tissue sensitivity to circulating vitamin D due to altered vitamin D receptor genotypes may play a role. Vitamin D receptor allelic polymorphisms, designated B/b, A/a, and T/t alleles correlate with bone mineral density. The risk of developing a vertebral fracture increased 2- to 3-fold with the presence of a T/t allele.[12] In CLD, deficiency of vitamin K is also seen, especially in cholestatic liver disease. Vitamin K is important in the formation of osteocalcin and osteonectin. Supplementation of vitamin K is associated with improvements in BMD.[13] Furthermore, vitamin K2 inhibits expression ligand (RANKL), tartrate resistant acid phosphatase (TRAP) activity, mononuclear cell formation, and also induces osteoclast apoptosis in vitro.[14]
OPG and RANKL: Osteoprotegerin (OPG), a member of the tumor necrosis factor receptor super family is produced by the liver and it inhibits osteoclast differentiation whereas receptor activator of nuclear factor Kb ligand (RANKL) plays a role in the differentiation and activation of osteoclasts by binding to its high affinity receptor (RANK) located on the surface of osteoclasts. Role of OPG in hepatic osteodystrophy is speculative; a decline in liver function may be associated with reduced production of OPG and increased osteoclastmediated bone resorption. Though contribution of the OPG/ RANKL system in osteoporosis and osteopenia in liver diseases including primary biliary cirrhosis is uncertain, since circulating OPG is increased and soluble RANKL is decreased, regardless of osteoporosis.[15] Thus, OPG/RANKL system is working in a different way in cirrhosis, which might be due to an increased RANK/RANKL affinity which is not measurable, and consumes part of total RANKL leaving smaller amount of measurable soluble RANKL to be assessed, which would explain its lower level in serum despite increased osteoporotic changes in bone.
Corticosteroid therapy is the primary therapy for autoimmune hepatitis and has been the mainstay of immunosuppression after liver transplantation. Prolonged steroid therapy results in clinically significant bone loss with an increase in fracture risk by greater than 2-fold.[16] Steroids exert a direct effect on bone cells by increasing osteoclastic activity by increasing IL1 and IL6 and decreasing differentiation, recruitment and life span of oteoblasts
Liver transplantation and Bone disease: - Following liver transplantation theres is an initial decline in BMD which gets stabilized within 6 months, followed by improvement. Early bone loss after liver transplantation is not only attributed to corticosteroids, but also to immunosuppressive agents such as the calcineurin inhibitors. Calcineurin inhibitors are used in conjunction with corticosteroids so the independent effects of these agents on bone metabolism in humans is difficult to ascertain. Additional medications used in the treatment of advanced liver disease, such as diuretics, anticoagulants, and chemotherapy also have deleterious effect on bone.[17]
Clinical presentation and diagnosis
Clinically these patients present with bone pains, backache, loss of height, fragility fractures and kyphosis/ scoliosis. For making a diagnosis of hepatic osteodystrophy, there should be documented liver disease. Various biochemical tests may be useful to ascertain calcium metabolism and gonadal hormone status: serum calcium, phosphate, thyroid function tests, intact parathyroid hormone, 25-hydroxyvitamin D, free testosterone (men), serum estradiol, and luteinizing hormone (women). Other tests include X-RAY, DEXA scan, QCT (quantitative computerized tomography), transcortical bone biopsy and biochemical markers of bone disease.
Hepatic osteodystrophy is characterized by:- (1) _ serum 25(OH)D and (2) _ 1,25-dihydroxyvitamin D (1,25[OH]2D3), (3) _ albumin, and (4) _ vitamin D binding protein required for transport of 25(OH)D to the kidneys.
Skeletal radiographs are useful adjuncts to bone mineral density measurements, as the risk of future vertebral fracture is predicted by the presence of preexisting spinal fractures. Dual Energy X-ray Absorptiometry (DEXA) is the widely used method to quantify BMD. It can be used for spine, hip, and forearm, the more common sites of fracture. A less expensive quantitative ultrasound may serve as a useful screening tool. Quantitative CT can be used but delivers a larger X-ray dose.
Indications for BMD testing are: - Chronic cholestasis, alcohol abuse, postmenopausal women with additional risk factors for osteoporosis, male hypogonadism, long-term corticosteroid therapy (more than 3 months), any patient with a fragility fracture, low body mass index and evaluation for transplantation. Contraindications for bone densitometry are pregnancy and recent gastrointestinal contrast studies and nuclear medicine tests.
WHO has defined normal bone density; osteopenia and osteoporosis based on the T-score that has become the standard for quantify fracture risk. (Table 2).
Risk of fracture increases two to threefold for each standard deviation (SD) decrease in BMD.[18] A T-SCORE is the number of standard deviations the bone mineral density measurement is above or below the YOUNG-NORMAL MEAN bone mineral density. A Z-SCORE is the number of standard deviations the measurement is above or below the AGEMATCHED MEAN bone mineral density.
If bone loss is exclusively caused by the normal process of ageing, the Z score is nearly zero. Secondary disease should be suspected when there is a significant negative deviation of Z score.[19]
BMD has a high specificity for fracture but a low sensitivity and so has not been advocated for population screening. Although MBD is a systemic condition, BMD assessment at one site correlates imperfectly with measurement of another site in the same patient, and screening for low BMD at one site can underestimate its frequency and severity. So assessment should be at three sites at second,third and fourth lumbar vertebra at femoral neck, and lower end of radius to assess both central and peripheral affection. Axial is affected earlier than peripheral in both cirrhotic and non
hepatic patients.
Advantages of T – scores are that it is unit less, is the basis for majority of osteoporosis guidelines and simple to use, Though disadvantages are that it depends on site measured, on technology, on reference database— population mean and standard deviation and also it only includes BMD information and not additional risk factors.
If patients have normal BMD, serial BMD monitoring should be done every 2-3 years, but In clinical conditions associated with a rapid bone loss such as in cholestatic patients with more than one risk factor for osteoporosis, and in those recently initiating high-dose corticosteroid therapies, the screening should be performed in a shorter interval of approximately one year.
Diagnosis of osteomalacia is by transcortical bone biopsy of rib or iliac crest; this test being invasive is not commonly done and treatment is often guided by biochemical indices only.
Biochemical markers of bone disease: - Markers of bone formation are procollagen propeptides of type 1 collagen, osteocalcin and bone isoenzyme of alkaline phosphatase. Markers of bone resorption are urinary excretion of deoxypyridinoline, pyridinoline and Type 1 collagen cross linked N-telopeptide. Levels of these markers are affected by the extent of hepatic fibrosis and none of these markers has been studied in patients with chronic liver disease. Hence, they cannot yet be recommended as a means of assessing bone loss and the risk of fracture in cirrhotic patients.
Management
Despite the introduction of methods to identify those with osteoporosis and despite effective treatment, a large ‘care gap’ continues to exist for these patients.
Interventions based on DEXA
T-score>-1 =normal bone density
Counsel all patients on risk factor reduction including:- Adequate consumption of calcium and vitamin D, Regular weight-bearing and muscle strengthening exercise, smoking cessation and limiting alcohol intake to <2 drinks daily. Besides this discontinue or substitute high risk medications and initiate therapy for patients on long term steroids.
T-score in between -1 and -2.5 = osteopenia
Counsel all patients on risk factor reduction, initiate therapy for women with T score below -2, Initiate therapy for women with one or more risk factors and a T score below -2, initiate therapy for any patient with a history of fragility fracture and initiate therapy for patients on long term steroids.
T-sore <2.5 =osteoporosis
Counsel all patients on risk factor reduction, initiate therapy, schedule routine follow up appointments to access compliance and effectiveness of prescribed interventions, repeat DEXA within 12-24 months and requires lifelong management.
Nutritional Therapy
Nutritional inadequacies can often be corrected through improved dietary habits and appropriate supplementation.
Some recommendations given by NIH are[20] :
1) Antioxidants vitamins-
· Vitamin A -essential for bone growth. Both increased as well as decreased levels of Vitamin A re detrimental, optimal dose not known.
· Vitamin C -is important in formation of collagen, recommended dose is 100-125mg/day
2) Zinc- it functions as a coenzyme essential for bone structure and strength. Low serum level of zinc has been related to osteoporosis. Excess intake of zinc interferes with copper absorption.
3) Iron -coenzyme involved in collagen synthesis.
4) Manganese -essential enzyme cofactor required for formation of healthy cartilage and bone. Adequate dose:- men 2-3mg/day; women 1.8mg/day.
5) Copper- essential as an enzyme cofactor required for normal bone metabolism and bone strength.
6) Proteins- Low protein diets result in decreased calcium absorption. High protein diets results in increased calciuria. It is controversial whether animal or vegetable source of protein is better, consuming 1-1.5g protein/kg body weight daily seems to be optimal for bone health.
7) Phosphorus- excess phosphorus interferes with calcium absorption; moreover its deficiency also reduces calcium absorption. its daily recommended dose is 700mg for adults> 30 years old. Daily intake >4000mg is not recommended.
8) Calcium- its daily adequate intake: Age 19-50 years – 1000 mg and >50 years – 1200 mg. The upper limit of safe daily intake is 2500mg.
9) Vitamin D- it is essential for calcium absorption, bone mineralization, and involved in bone turnover. Casual sunlight exposure is best source. Its daily intake is: - Age 19-50 years - 200IU (5µg), 51-69 years - 400IU (10µg) and e”70 years - 600IU (15µg). Safe upper limit is 2000 IU daily
Vitamin D deficiency is increasingly common in the general population and very common among the elderly.[21] Chronic Vitamin D deficiency is the principle cause of osteomalacia. Although calcium and vitamin D supplements are recommended, there are no unequivocal data confirming the efficacy of these supplements for preventing bones loss in patients who have liver disease.
Specific therapies
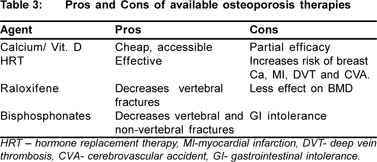
The options available are: hormone replacement therapy, raloxifene, testosterone, bisphosphonates, calcitonin, sodium fluoride, strontium ranelate, teriparatide.
Hormone replacement therapy(HRT) is well-known to preserve and increase BMD prevents hip fractures. However, it increases breast cancer and cardiovascular risk. It has been used as standard therapy for postmenopausal osteoporosis with a propensity for hepatotoxocity in patients with chronic liver disease. It is used as a sequential combination therapy, continuous combination therapy, or estrogens alone in women who have had a hysterectomy. Boone and colleagues[22] have demonstrated in a placebo controlled trial that the percentage of bone loss in femoral neck was significantly lower in actively treated patients than in those receiving placebo. The recommended duration of HRT is 5–10 years. Despite optimistic results, HRT currently is not considered the most appropriate treatment as there are other efficacious nonhormonal agents, such as bisphosphonates, with lesser side effects for the treatment of osteoporosis associated with CLD.
Raloxifene, a SERM (selective estrogen receptor modulator) has been approved for prevention and treatment of post menopausal osteoporosis. It has shown to increase BMD at spine and hip and to reduce vertebral fractures, but effect on hip and other fractures not yet proven. Levy et al[23] in a pilot study including 9 postmenopausal women who had PBC have shown that compared with baseline, lumber BMD improved significantly after 1 year of therapy but not in matched controls and no significant effect was observed in femoral neck bone density in either group. Before recommending this drug for more widespread use larger controlled trials are warranted.
Testosterone replacement in hypogonadal men leads to increases in BMD,[24] the potential risk/benefit must be discussed with individuals before starting replacement therapy. Till now, no studies of the effects of testosterone replacement in patients with CLD has been done. Moreover, it might increase the risk of HCC( hepatocellular carcinoma).
Bisphosphonates are phosphate derivatives that attach to the surface of bone and thereby prevent osteoclastmediated resorption. They reliably increase BMD and reduce fracture risk. These include oral agents like alendronate, etidronate, risedronate and parental agents like pamidronate, zoledronate. Bisphosphonates have been shown to reduce vertebral, hip, and other fractures (new fractures reduced by 40 - 50%). But its use limited by esophageal symptoms and inconvenience. Among bisphosphonates, alendronate is more effective than etidronate.[25] Once-weekly Alendronate (70mg) in primary biliary cirrhosis (PBC) indicates that this regimen is more effective and has a better tolerability profile than daily dosing.[26] Bisphosphonates should be taken on an empty stomach in the morning, 0.5–2 hours before consumption of food and other drugs, and at a different time to calcium supplements as calcium binds and inactivates bisphosphonates. A randomized controlled trial in 99 adults awaiting OLT (orthotropic liver transplantation) did not find any benefit of a single preoperative infusion of pamidronate on BMD or fractures, although bone histomorphometry showed that the increase in bone resorption usually seen after OLT was partially suppressed by treatment.[33] There is no information on the effects of other more potent bisphosphonates such as zoledronic acid in CLD.
Calcitonin: Calcitonin as nasal spray is approved for treatment of PMO (post menopausal osteoporosis) in women > 5 yrs postmenopausal. It acts by inhibiting osteoclast activity and number. Its effect on BMD is less than bisphosphonates, but decrease in new vertebral fractures is almost as good. It has no proven reduction in non-vertebral fractures (hip and others). however, effect of this antiresorptive agent in patients who have CLD is not clear.
A pilot study reported that the combined administration of calcitonin, calcium, and vitamin D over 12 months was associated with lower bone loss than in untreated patients. Apparently the long term effects were more favorable in patients who had osteopenia.[27] Another study has reported that the administration of calcitonin over 6 months was unable to prevent the loss of trabecular bone, although it should be taken into account that patients were treated for a short period of time.[28]
Sodium fluoride increases bone formation and is used in certain forms of osteoporosis, although in recent years it has been overtaken by antiresorptive drugs.
The effects of newer agents such as strontium ranelate[29] or anabolic therapy with synthetic N-terminal portion of PTH[30], human recombinant PTH peptide 1-34 (teriparatide)[31] or 1-84 parathyroid hormone32 used in postmenopausal osteoporosis also deserves further investigation in patients who have CLD. (Table 3)
Conclusions
Hepatic osteodystrophy is under-recognized and less attended complication of CLD. Newer diagnostic modalities have improved the detection of HO and Vitamin D repletion, calcium supplementation and bisphosphonates seem promising therapies.
References
1. Rouillard S, Lane NE. Hepatic osteodystrophy. Hepatology. 2001;33:301–7.
2. Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts and prospects. J Clin Invest. 2005;115:3318–25.
3. Sanchez AJ, Aranda-Michel J. Liver disease and osteoporosis. Nutr Clin Pract. 2006;21:273–8.
4. Cijevschi C, Mihai C, Zbranca E, Gogalniceanu P. Osteoporosis in liver cirrhosis. Rom J Gastroenterol. 2005;14:337–41.
5. Pares A, Guanabens N, Alvarez L, De Osaba MJ, Oriola J, PonsF, et al. Collagen type Ialpha1 and vitamin D receptor gene polymorphisms and bone mass in primary biliary cirrhosis. Hepatology. 2001;33:554–60.
6. Newton J, Francis R, Prince M, James O, Bassendine M, Rawlings D, et al. Osteoporosis in primary biliary cirrhosis revisited. Gut. 2001;49:282–7.
7. Guanabens N, Pares A, Ros I, Caballerýa J, Pons F, Vidal S, et al. Severity of cholestasis and advanced histological stage but not menopausal status are the major risk factors for osteoporosis in primary biliary cirrhosis. J Hepatol. 2005;42:573–7.
8. Monegal A, Navasa M, Guanabens N, Peris P, Pons F, Martinez de Osaba MJ, et al. Osteoporosis and bone mineral metabolism disorders in cirrhotic patients referred for orthotopic liver transplantation. Calcif Tissue Int. 1997;60:148–54.
9. Ninkovic M, Skingle SJ, Bearcroft PW, Bishop N, Alexander GJ, Compston JE. Incidence of vertebral fractures in the first three months after orthotopic liver transplantation. Eur J Gastroenterol Hepatol. 2000;12:931–5.
10. Eriksen EF, Kassem M, Langdahl B. Growth hormone, insulin-like growth factors and bone remodelling. Eur J Clin Invest. 1996;26:525–34.
11. Smith DL, Shire NJ, Watts NB, Schmitter T, Szabo G, Zucker SD. Hyperbilirubinemia is not a major contributing factor to altered bone mineral density in patients with chronic liver disease. J Clin Densitom. 2006;9:105–13.
12. Springer JE, Cole DE, Rubin LA, Cauch-Dudek K, Harewod L, Evrovski J, et al. Vitamin D -receptor genotypes as independent genetic predictors of decreased bone mineral density in primary biliary cirrhosis. Gastroenterology. 2000;118:145–51.
13. Cockayne S, Adamson J, Lanham-new S, Shearer MJ, Gilbody S, Torgerson DJ. Vitamin K and prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:1256–61.
14. Sasaki N, Kusano E, Takahashi H, Ando Y, Yano K, Tsuda E, et al. Vitamin K2 inhibits glucorticoid-induced bone loss partly by preventing the reduction of osteoprotegrin (OPG). J Bone Miner Metab. 2005;23:41–7.
15. Pare´s A, Guan˜abens N, Alvarez L, Monegal A, Caballerý´a L, Ozalla D, et al. High osteoprotegerin and low RANKL levels in primary biliary cirrhosis. Relationship with the severity of liver disease but not with osteoporosis. Hepatology (abstract). 2004;40:463.
16. Adler RA, Rosen CJ. Glucocorticoids and osteoporosis. Endocrinol Metab Clin North Am. 1994;23:641–54.
17. Cohen A, Ebeling P, Sprague S, et al. Transplantation osteoporosis. In: Favus M, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. 6th edition. Washington, DC: American Soceity for bone and mineral research; 2006.p.302–9.
18. Marshall D, Jonhell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254–9.
19. Licata AA. Diagnosing primary osteoporosis: it’s more than a T score. Cleve Clin J Med. 2006;73:473–6.
20. National Institutes of Health Osteoporosis and Related Bone Disease- National Resource Centre. Health topics: other nutrients and bone Health. Bethesda (MD). 2004.
21. Holick MF, et al. Vitamin D. In: Shils ME, Olsen JA, Shine M, editors. Modern nutrition in health and disease. 9th edition. Philadelphia: Lippincott Williams & Wilkins; 1999.p.329–46.
22. Boone RH, Cheung AM, Girlan LM, Healthcote EJ. Osteoporosis in primary biliary cirrhosis: a randomized trial of the efficacy and feasibility of estrogen/progestin. Dig Dis Sci. 2006;51:1103–12.
23. Levy C, Harnois DM, Angulo P, Jorgensen R, Lindor KD. Raloxifene improves bone mass in osteopenic women with CLD: results of a pilot study. Liver Int. 2005;25:117–21.
24. Behre HM, von Eckardstein S, Kliesch S, et al. Long-term substitution therapy of hypogonadal men with transscrotal testosterone over 7–10 years. Clin Endocrinol. 1999;50:629–35.
25. Guanabens N, Pares A, Ros I, Alvarez L, Pons F, Caballería L, et al. Alendronate is more effective than etidronate for increasing bone mass in osteopenic patients with primary biliary cirrhosis. Am J Gastroentrol. 2003;98:2268–74.
26. Zein C, Jorgensen R, Clarke B, Wenger DE, Keach JC, Angulo P, et al. Alendronate improves bone mineral density in primary biliary cirrhosis.: a randomized placebo-controlled trial. Hepatology. 2005;42:762–71.
27. Floreani A, Zappala F, Fries W, Naccarato R, Plebani M, D’Angelo A, et al. A 3-year pilot study with 1,25-dihydroxyvitamin D, calcium and calcitonin for severe osteodystrophy in primary biliary cirrhosis. J Clin Gastroenterol. 1997;24:239–44.
28. Camisasca M, Crosignani A, Battezzati PM, Albisetti W, Grandinetti G, Pietrogrande L, et al. Parenteral calcitonin for metabolic bone disease associated with primary biliary cirrhosis. Hepatology. 1994;20:633–7.
29. Meunier PJ, Roux C, Seeman E, Ortolani S, Badurski JE, Spector TD, et al. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med. 2004;350:459–68.
30. Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effects of parathyroid hormone(1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–41.
31. Borba VZ, Mañas NC. The use of PTH in the treatment of osteoporosis. Arq Bras Endocrinol Metabol. 2010;54:213–9.
32. Greenspan SL, Bone HG, Ettinger MP, Hanley DA, Lindsay R, Zanchetta JR, et al. Effect of recombinant human parathyroid hormone (1-84) on vertebral fracture and bone mineral density in post menopausal women with osteoporosis: a randomized trial. Ann Intern Med. 2007;146:326–39.
33. Ninkovic M, Love S, Tom BD, Bearcroft PW, Alexander GJ, et al. Lack of effect of intravenous pamidronate on fracture incidence and bone mineral density after orthotropic liver transplantation. J. Hepatol. 2002;37:93-100.
|
|
|
 |
|
|