|
|
|
|
 |
 |
| |
 |
|
|
Case Report |
|
|
|
|
|
Keywords :
|
|
|
Chethana Kanaparthi1, Shashideep Singhal1, Carl Guillaume2, Shweta Sharma2, Sury Anand1
Gastroenterology, The Brooklyn Hospital Center,1
Brooklyn,
Gastroenterology, St. Barnabas Hospital,2 Bronx,
New York, USA
Corresponding Author:
Dr. Shashideep Singhal
Email: sdsinghal@gmail.com
DOI:
http://dx.doi.org/
48uep6bbphidvals|474 48uep6bbph|2000F98CTab_Articles|Fulltext Acute gastrointestinal necrosis (AGN) can result from multitude of local or systemic factors. The distribution of necrosis is likely to depend on the site of the insult and/or the vascular supply of the affected bowel. A rare syndrome of “Acute esophageal necrosis” (AEN) or “Black esophagus” (BEs) has been described earlier referring to black discoloration of esophageal mucosa seen on endoscopic exam.[1,2,3] The reported incidence of AEN is 0.008% to 0.2% and the etiology is assumed to be largely multifactorial.[3] Ischemia has been proposed to be the commonest suspected cause of AEN, but the preferential involvement of esophagus despite having rich segmental and intramural blood supply needs further explanation. We herein, report a case of AGN presenting as black esophagus. With anextensive review of literature we have elucidated the risk factors, mechanisms of gut injury, clinical presentation,
treatment options and prognosis of the syndrome.
Case Report
A 76-year-old female was admitted with a three day history of nausea, vomiting and epigastric pain, followed by coffee ground emesis and malena on the day of admission. She had no history of gastroesophageal reflux, peptic ulcer disease, ingestion of corrosives, alcohol or NSAID medication. Her comorbidities included coronary artery disease, chronic congestive heart failure, stroke, diabetes mellitus type II, hypertension, dyslipidemia, asthma and age related dementia. Her medications were clopidrogel, ranitidine, insulin, losartan, furosemide, simvastatin and donepezil.
Examination revealed an obese female in mild distress, afebrile, with a mean blood pressure 66 mmHg, pulse rate 101/ min, respiratory rate 16/min and no pallor or icterus. There were no oral mucosal lesions and abdominal exam revealed epigastric tenderness. Rectal exam showed black haem positive stool. Patient had normal hemoglobin with leucocytosis and neutrophilia (WBC 25,100 /mm3, neutrophils 89.5%). Biochemical tests showed hyperglycemia (glucose 475 mg/dL), normal electrolytes, absence of anion gap or ketones and presence of acute renal failure with BUN 66 mg/dL, creatinine 1.8 mg/dL.
Liver function tests showed mild hyperbilirubinemia: total bilirubin 1.9 mg/dL, direct bilirubin 0.5 mg/dL, normal aminotransferases and mild elevation in alkaline phosphatase 286 IU/L, prothrombin time 17.4 sec and INR 1.8. CPK, troponin I, amylase and lipase were in normal range. A computed tomographic (CT) scan of abdomen with oral contrast revealed sliding hiatal hernia with evidence of gastroesophageal reflux and thickening of duodenum with associated mesenteric fat stranding suggestive of duodenitis. There was no evidence of obstruction. Notably, there were atherosclerotic calcifications of abdominal aorta and its branches.
An upper gastrointestinal endoscopy (UGIE) showed black esophagus; erythematous upper third of esophagus, with thickening of esophageal mucosa and circumferential areas of black necrosis, ulceration and exudates in the middle and lower third of esophagus and clear cut margins at gastro-esophageal junction (Figure 1a & b). Gastroscopy revealed multiple tiny shallow ulcers highly suggestive of ischemia (Figure 1c). Similar to esophagus, there were patches of necrotic, ulcerated and edematous mucosa in the bulb and 2nd part of duodenum (Figure 1d). No active bleeding site was identified. Pathology findings of esophagus were that of necrotic tissue with inflammatory cell exudates and the duodenal specimen had evidence of acute and chronic inflammation. Stains for viruses and fungi were negative.
Patient was kept NPO, managed with intra venous fluids to maintain perfusion, strict glycemic control and intra venous proton pump inhibitor (esmoprazole). In view of elevated WBC count the patient was also started on piperacillin-tazobactam. Follow-up stool studies were negative for WBC’s and ovaparasite, and the stool and blood cultures did not show any growth of pathogens. She significantly improved over next few days and was started on clear liquid diet and was switched to oral esmoprazole. Further course was altered by an episode of malena on day 6 of admission with a drop in hemoglobin by 4 gm/dL which needed blood transfusion. Patient was restarted on intravenous esmoprazole and endoscopy was planned. A repeat UGIE showed healing ischemic injury of esophagus, normal stomach and erythematous duodenal mucosa with whitish plaques. Colonoscopy revealed intact colonic mucosa coated with slough and old blood suggestive of reperfusion injury. Patient was started on clear liquid diet after endoscopy and gradually advanced. Her hemoglobin remained stable, there were no further symptoms and she was discharged.
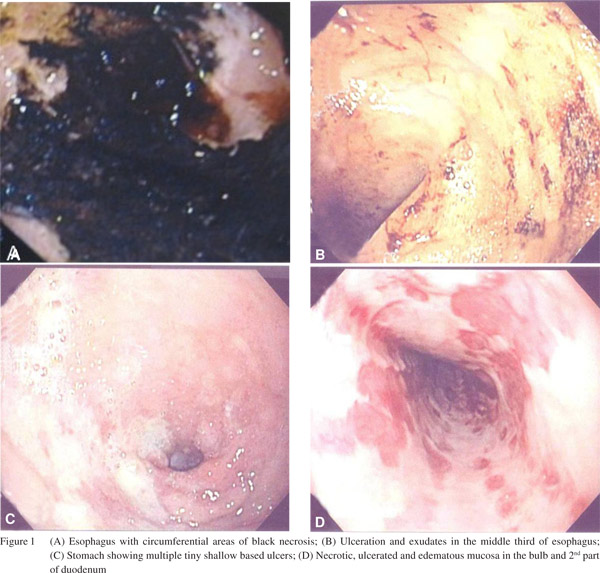 Discussion
Black esophagus or acute esophageal necrosis is a rare condition reported earlier in patients with multiple comorbidities and largely considered to be interplay between ischemic injury and effect of acid reflux.[3,4] The metabolic disturbances leading to the syndrome are likely to affect other parts of the gastrointestinal tract which has not been described in previous reports.[1,2,3,4
Discussion
Black esophagus or acute esophageal necrosis is a rare condition reported earlier in patients with multiple comorbidities and largely considered to be interplay between ischemic injury and effect of acid reflux.[3,4] The metabolic disturbances leading to the syndrome are likely to affect other parts of the gastrointestinal tract which has not been described in previous reports.[1,2,3,4]
Incidence and risk factors
AEN is a rare disorder with an estimated incidence of zero to 0.2%.[2,5] The condition has been reported mostly in elderly with a sex ratio favoring men (M:F = 4:1).[2,3] While ischemia and acid reflux have been incriminated as the pathophysiological mechanisms of this disease by most authors, the risk factors are diverse and shown in Table 1.[1,2,3,4,5]
AGN or AEN?
All proposed theories of AEN point towards an imbalance in the perfusion and the physiological defense mechanisms in response to reflux related injury.6 Our patient had multiple risk factors which are likely to have caused systemic insults and a diffuse injury in the gastrointestinal (GI) tract. There was evidence of shallow ulcerations in stomach as well as patches of necrotic, ulcerated and edematous mucosa in duodenum similar to that seen in esophagus. Involvement of duodenum in form of duodenitis or ulcers has been reported earlier.[7] Colon examination also revealed evidence of reperfusion injury which has not been described in any previous reports. These findings support involvement of complete GI tract presenting as AGN in the patient. The typical black appearance of esophagus and duodenum can be attributed to the effect of acid on hypoperfused gut wall, as described earlier.[4,6] Despite the multiple proposed hypothesis of gut injury the mechanism of diffuse homogenous involvement in large proportion of cases remains unclear.
 Clinical presentation and laboratory findings
The commonest manifestation reported in majority (>80%) of cases and as seen in our case is upper GI bleed which can present as hematemesis, coffee ground emesis or melena.[2,3,4,5] Other symptoms such as epigastric pain, dysphagia, vomiting and anemia are usually associated with GI bleed but can be the sole manifestation.[6,8] Evidence of renal insufficiency was also present in our patient and has been reported in one third of cases.[2,3] Laboratory findings occasionally reveal leucocytosis, while hepatic function abnormalities have also been reported.[9,10]
Role of imaging
Chest radiograph can be helpful to exclude presence of esophageal perforation. It can also aid in visualizing aortic dissection or mediastinal masses which have been incriminated as etiology of BE.[8] Thickening of esophagus or duodenum can be seen on CT scan. Mesenteric fat stranding can be a sign of inflammation, while evidence of mediastinitis can guide appropriate timely intervention. Compromise in the blood supply of gut can be determined by use of CT angiogram.
Role of endoscopy
Endoscopy is needed for diagnosis and is considered safe. The characteristic endoscopic appearance of black diffusely necrotic esophageal mucosa, with or without exudates is diagnostic.[1] Classic circumferential involvement of the distal one-third of esophagus with clear demarcation at the gastroesophageal junction is relatively uncommon, rather BE tends to show diffuse involvement in more than half of cases described.[2] Selective distal esophageal involvement can aid in differentiating AEN from corrosive injury of esophagus. Other conditions which can mimic endoscopic appearance of BE are quinine ingestion, acanthosis nigricans, melanosis and pseudomelanosis of esophagus.
Presence of gastric and duodenal involvement in form of erosions or ulcerations in our case and also in two thirds of the cases reported in literature indicates role of gastric acid on the ischemic mucosa leading to AGN.[2,3] Involvement of lower bowel associated with AEN has not been reported earlier. Our patient had evidence of reperfusion injury in the distal bowel suggesting a diffuse involvement of GI tract.
Endoscopy can also be helpful for follow up of patients with AGN. Healing of mucosa can be evident as early as day 7 when our patient had appearance of whitish exudates. In most patients esophageal lesions are reported to resolve completely by 8-10 weeks. Endoscopy can also aid in diagnosis and treatment of complications.
Histopathology
Biopsy usually reveals necrotic mucosa with or without viable epithelium along with evidence of inflammatory cells. The extent of necrosis is usually mucosal, frequently submucosal and rarely involves muscularis mucosa.[11,12] The presence of acute on chronic inflammatory cells in duodenal biopsy is possibly due to increased acid secretion in these patients leading to chronic inflammation. Biopsy can additionally help to exclude infectious causes.
Complications
Esophageal stenosis or stricture formation have been reported in 10-15% of patients and can present from one week to two months after diagnosis.[13] Strictures usually respond to multiple dilations but might need surgery. Other complications such as esophageal perforation, mediastinitis and abscess formation, are reported in approximately 6% patients.[2]
Management
AGN being a rare syndrome there are no studies to determine the most appropriate treatment. Patients with AGN usually have multiple co-morbidities leading to the pathogenesis of this syndrome. Treating the underlying cause is of utmost importance. Ischemic phenomena have been the suggested etiologies in a majority of cases hence maintaining perfusion should be the primary goal. All patients should receive fluid resuscitation and blood transfusion in presence of anemia. Measures to control gastric acid have been used universally.[3] Intra venous proton pump inhibitors cause better acid suppression in comparison to H2 blockers and should be preferred.[14] Combined use of sucralfate with acid suppressing therapy has been reported to be useful.[2,3]
The role of antibiotics in management of AEN is not clear. Empiric broad spectrum antibiotics can be used in sick patients with multiple comorbidities and should be discontinued if no source of infection is identified. In our patient feeding was withheld for the initial few days and was gradually resumed as the patient was able to tolerate clear liquids. However there is a risk of further bleeding as was seen in our case and patients should be monitored closely. Inability to resume feeding may warrant parenteral nutrition in some cases.
Outcome
AEN has a grave prognosis with mortality rates as high as 36%.[2] Fortunately our patient survived despite having multiple co-morbidities and renal failure. Shock is the commonest cause of death and can be septic, cardiogenic or hypovolemic.
Conclusion
AGN is a rare syndrome of ischemic injury of GI tract with selective involvement of esophagus presenting as black esophagus. The etiology is multifactorial and hence treatment should target underlying cause in addition to fluid resuscitation and acid suppression. Timely diagnosis and treatment is crucial to improve survival in these patients.
References
Clinical presentation and laboratory findings
The commonest manifestation reported in majority (>80%) of cases and as seen in our case is upper GI bleed which can present as hematemesis, coffee ground emesis or melena.[2,3,4,5] Other symptoms such as epigastric pain, dysphagia, vomiting and anemia are usually associated with GI bleed but can be the sole manifestation.[6,8] Evidence of renal insufficiency was also present in our patient and has been reported in one third of cases.[2,3] Laboratory findings occasionally reveal leucocytosis, while hepatic function abnormalities have also been reported.[9,10]
Role of imaging
Chest radiograph can be helpful to exclude presence of esophageal perforation. It can also aid in visualizing aortic dissection or mediastinal masses which have been incriminated as etiology of BE.[8] Thickening of esophagus or duodenum can be seen on CT scan. Mesenteric fat stranding can be a sign of inflammation, while evidence of mediastinitis can guide appropriate timely intervention. Compromise in the blood supply of gut can be determined by use of CT angiogram.
Role of endoscopy
Endoscopy is needed for diagnosis and is considered safe. The characteristic endoscopic appearance of black diffusely necrotic esophageal mucosa, with or without exudates is diagnostic.[1] Classic circumferential involvement of the distal one-third of esophagus with clear demarcation at the gastroesophageal junction is relatively uncommon, rather BE tends to show diffuse involvement in more than half of cases described.[2] Selective distal esophageal involvement can aid in differentiating AEN from corrosive injury of esophagus. Other conditions which can mimic endoscopic appearance of BE are quinine ingestion, acanthosis nigricans, melanosis and pseudomelanosis of esophagus.
Presence of gastric and duodenal involvement in form of erosions or ulcerations in our case and also in two thirds of the cases reported in literature indicates role of gastric acid on the ischemic mucosa leading to AGN.[2,3] Involvement of lower bowel associated with AEN has not been reported earlier. Our patient had evidence of reperfusion injury in the distal bowel suggesting a diffuse involvement of GI tract.
Endoscopy can also be helpful for follow up of patients with AGN. Healing of mucosa can be evident as early as day 7 when our patient had appearance of whitish exudates. In most patients esophageal lesions are reported to resolve completely by 8-10 weeks. Endoscopy can also aid in diagnosis and treatment of complications.
Histopathology
Biopsy usually reveals necrotic mucosa with or without viable epithelium along with evidence of inflammatory cells. The extent of necrosis is usually mucosal, frequently submucosal and rarely involves muscularis mucosa.[11,12] The presence of acute on chronic inflammatory cells in duodenal biopsy is possibly due to increased acid secretion in these patients leading to chronic inflammation. Biopsy can additionally help to exclude infectious causes.
Complications
Esophageal stenosis or stricture formation have been reported in 10-15% of patients and can present from one week to two months after diagnosis.[13] Strictures usually respond to multiple dilations but might need surgery. Other complications such as esophageal perforation, mediastinitis and abscess formation, are reported in approximately 6% patients.[2]
Management
AGN being a rare syndrome there are no studies to determine the most appropriate treatment. Patients with AGN usually have multiple co-morbidities leading to the pathogenesis of this syndrome. Treating the underlying cause is of utmost importance. Ischemic phenomena have been the suggested etiologies in a majority of cases hence maintaining perfusion should be the primary goal. All patients should receive fluid resuscitation and blood transfusion in presence of anemia. Measures to control gastric acid have been used universally.[3] Intra venous proton pump inhibitors cause better acid suppression in comparison to H2 blockers and should be preferred.[14] Combined use of sucralfate with acid suppressing therapy has been reported to be useful.[2,3]
The role of antibiotics in management of AEN is not clear. Empiric broad spectrum antibiotics can be used in sick patients with multiple comorbidities and should be discontinued if no source of infection is identified. In our patient feeding was withheld for the initial few days and was gradually resumed as the patient was able to tolerate clear liquids. However there is a risk of further bleeding as was seen in our case and patients should be monitored closely. Inability to resume feeding may warrant parenteral nutrition in some cases.
Outcome
AEN has a grave prognosis with mortality rates as high as 36%.[2] Fortunately our patient survived despite having multiple co-morbidities and renal failure. Shock is the commonest cause of death and can be septic, cardiogenic or hypovolemic.
Conclusion
AGN is a rare syndrome of ischemic injury of GI tract with selective involvement of esophagus presenting as black esophagus. The etiology is multifactorial and hence treatment should target underlying cause in addition to fluid resuscitation and acid suppression. Timely diagnosis and treatment is crucial to improve survival in these patients.
References
- Moretó M, Ojembarrena E, Zaballa M, Tánago JG, Ibánez S. Idiopathic acute esophageal necrosis: not necessarily a terminal event. Endoscopy. 1993;25:534–8.
- Gurvits GE, Shapsis A, Lau N, Gualtieri N, Robilotti JG. Acute esophageal necrosis: a rare syndrome. J Gastroenterol. 2007;42:29–38.
- Grudell AB, Mueller PS, Viggiano TR. Black esophagus: report of six cases and review of the literature, 1963-2003. Dis Esophagus. 2006;19:105–10.
- Burtally A, Gregoire P. Acute esophageal necrosis and low-flow state. Can J Gastroenterol. 2007;21:245–7.
- Katsinelos P, Pilpilidis I, Dimiropoulos S, Paroutoglou G, Kamperis E, Tsolkas P, et al. Black esophagus induced by severe vomiting in a healthy young man. Surg Endosc. 2003;17:521.
- Ben Soussan E, Savoye G, Hochain P, Hervé S, Antonietti M, Lemoine F, et al. Acute esophageal necrosis: a 1-year prospective study. Gastrointest Endosc. 2002;56:213–7.
- Lacy BE, Toor A, Bensen SP, Rothstein RI, Maheshwari Y. Acute esophageal necrosis: report of two cases and a review of the literature. Gastrointest Endosc. 1999;49:527–32.
- Reichart M, Busch OR, Bruno MJ, Van Lanschot JJ. Black esophagus: a view in the dark. Dis Esophagus. 2000;13:311–3.
- Sãftoiu A, Cazacu S, Kruse A, Georgescu C, Comãnescu V, Ciurea T. Acute esophageal necrosis associated with alcoholic hepatitis: is it black or is it white? Endoscopy. 2005;37:268–71.
- Khan AM, Hundal R, Ramaswamy V, Korsten M, Dhuper S. Acute esophageal necrosis and liver pathology, a rare combination. World J Gastroenterol. 2004;10:2457–8.
- Jacobsen NO, Christiansen J, Kruse A. Incidence of oesophageal necrosis in an autopsy material. APMIS. 2003;111:591–4.
- Tsokos M, Herbst H. Black oesophagus: a rare disorder with potentially fatal outcome. A forensic pathological approach based on five autopsy cases. Int J Legal Med. 2005;119:146–52.
- Kim YH, Choi SY. Black esophagus with concomitant candidiasis developed after diabetic ketoacidosis. World J Gastroenterol. 2007;13:5662–3.
- Ghassemi KA, Kovacs TO, Jensen DM. Gastric acid inhibition in the treatment of peptic ulcer hemorrhage. Curr Gastroenterol Rep. 2009;11:462–9.
|
|
|
 |
|
|