|
|
|
|
 |
 |
| |
 |
|
|
Original Articles |
|
|
|
|
|
Keywords :
NAFLD; uric acid; insulin resistance; prediabetes. |
|
|
|
IA Hossain1, MO Faruque2, S Akter1, FR Bhuiyan1, MK Rahman3, L Ali1
1Department of Biochemistry & Cell Biology, Bangladesh University of Health Sciences, 2Department of Physiology & Molecular Biology, Bangladesh University of Health Sciences, 3Department of Biochemistry & Molecular Biology, University of Dhaka, Bangladesh.
Corresponding Author:
Liaquat Ali
Email: vc@buhs-edu.org
DOI:
http://dx.doi.org/10.7869/tg.334
Abstract
Background: Nonalcoholic fatty liver disease (NAFLD) is a hepatic manifestation of insulin resistance and serum uric acid (SUA) levels seemed to be elevated during this disorder. There is a paucity of data regarding the association of SUA with NAFLD in prediabetes. In this context, the present study has been undertaken to investigate this association.
Methods: In a cross-sectional analytical design, a total of 110 prediabetic subjects [M/F; 63/47, age in ranges, 45 (25-68)] were recruited in the study and divided into non NAFLD (n = 62) and NAFLD (n = 48) group after examined with ultrasonogram. Insulin resistance (HOMA-IR) was calculated by homeostasis model assessment.
Results: NAFLD subjects had significantly higher levels of SUA compared to non NAFLD subjects (6.10 ± 1.42 vs. 5.38 ± 1.14, p = 0.004). They also had significantly higher levels of HOMA-IR (2.4 ± 1.09 vs. 1.4 ± 0.45, p < 0.001). In binary logistic regression analysis, HbA1c (OR = 3.505, p = 0.002), SUA (OR = 1.514, p = 0.023) and HOMA-IR (OR = 1.478, p = 0.029) were found to be significant determinants of NAFLD after adjusting the effects of BMI and triglyceride (TG). In multiple linear regression analysis, SUA showed significant positive association with HOMA-IR (ß = 0.355, p = 0.027) and TG (ß = 0.325, p = 0.033) after adjusting the effects of BMI and HbA1c.
Conclusions: Increased levels of serum uric acid are significantly associated with NAFLD and this association seemed to be mediated by insulin resistance among prediabetic subjects.
|
48uep6bbphidcol2|ID 48uep6bbphidvals|1715 48uep6bbph|2000F98CTab_Articles|Fulltext Introduction
Nonalcoholic fatty liver disease (NAFLD) comprises a spectrum of pathologic conditions including simple steatosis, steatohepatitis and cirrhosis, influences approximately 20-30% of the general population and its prevalence is increasing worldwide. 1 NAFLD is commonly associated with obesity and insulin resistance, which are closely related to a cluster of other metabolic abnormalities, such as hypertriglyceridemia and hyperuricemia. 2 In recent years, an association between elevated serum uric acid (SUA) concentrations and NAFLD has been reported. 3 Prior epidemiological studies showed this association with some pathological processes included insulin resistance, oxidative stress, and systemic inflammation, 4,5 which are all considered as important risk factors for the development or progression of NAFLD. Consistent with a link between uric acid and NAFLD, Lonardo et al firstly described an association between NAFLD and SUA levels in a small case-control study of Italian patients with ultrasound-diagnosed NAFLD.6 Although several studies have suggested that SUA is significantly associated with NAFLD and its elevation is an independent risk factor for NAFLD, 3,7 however, the underlying mechanism(s) has not yet been clarified. Some studies have reported that patients with NAFLD have higher SUA levels than healthy controls. 6,8
SUA is the primary end product of purine metabolism in humans, and its levels are strictly controlled by the balance between production and excretion. Since UA is a biologically inert substance and possibly has anti-inflammatory role because of its function as an antioxidant. 9 However, accumulating evidence suggests that increased SUA levels associates with the development of NAFLD that depends on ‘‘two-hit’’ theory. 3 According to the ‘first hit’ theory, the accumulation of excessive fat in the liver due to insulin resistance that promotes lipolysis of peripheral adipose tissue and increases free fatty acid deposition into the hepatocytes leading to the development of NAFLD that increases UA synthesis and reduces the rennin angiotensin system thereby decreasing the renal UA excretion. The ‘second hit’ is the process where elevated levels of SUA causes the hepatocytes more vulnerable to further damage associates with oxidative stress, lipid peroxidation, inflammation and hepatic injury. 10
Prior epidemiological studies have shown that high SUA levels are also associated with altered glucose metabolism by decreasing the insulin stimulated glucose uptake in liver and muscle tissue. 5 Reduction of insulin signaling cascade in these tissues resulted the development of metabolic syndrome and its component like obesity, glucose intolerance and type 2 diabetes. The exact mechanism by which increased SUA levels affect glucose metabolism in prediabetic subjects is still controversial. Some studies reported that SUA is a biologically inert substances and it possess the antioxidant properties by protecting the cell from inflammation. 10 However, excess accumulation of SUA increases the lipid peroxidation thereby generating reactive oxygen species (ROS), induces endothelial dysfunction and systemic inflammation- all are risk factors developed from insulin resistance in liver, muscle and adipose tissue. 11 The combined effects of increased levels of SUA and insulin resistance plays crucial role in the pathogenesis of impaired glucose regulation. Since NAFLD is a hepatic manifestation closely related to elevated levels of SUA and insulin resistance, this association has been studied in several studies among T2DM subjects. 12,13 Compared with non diabetic subjects, T2DM are believed to have an increased risk of developing NAFLD, but the true prevalence of the disorder among prediabetic subjects has not been systematically assessed. In addition, so far, population-based data to determine whether SUA and insulin resistance levels are associated with NAFLD in different stages of impaired glucose regulation, in particular prediabetic states, is scarce. Therefore, the present study was set out to investigate the possible association between serum uric acid levels and insulin resistance with NAFLD having prediabetes.
Patients and Methods
Study design and subjects
A cross-sectional study was conducted among the prediabetic subjects to evaluate the relationship between the SUA levels and insulin resistance with the development of NAFLD. The study enrolled 110 (one hundred and ten) predaibetic subjects attending the BIHS Hospital, Darussalam, Dhaka, Bangladesh, in the period between March 2012 and October 2013. After an overnight fast of at least 8-10 hours, all nondiabetic participants underwent a standard 75-g oral glucose tolerance test (OGTT).We screened 279 (two hundred and seventy nine) subjects of which 14.3% (n = 40, M/F 21/19) was normal glucose tolerance (NGT), 15.8% (n = 44, M/F 30/14) was impaired fasting glucose (IFG), 29% (n = 81, M/F 37/44) was impaired glucose tolerance (IGT), 12.2% (n = 34, M/F 22/12) was combined IFG-IGT and 28.7% (n = 80, M/F 48/32) was newly diagnosed type 2 diabetes (NDD). Prediabetes and type 2 diabetes (T2DM) were defined according to the 2006 WHO diagnostic criteria based on both fasting and 2-hglucose values. 14 Subjects suffering from any systemic illness like acute and severe septic conditions, cardiac disease, hepatic, renal, respiratory failure, stroke and type 1 diabetes, those taking drugs that significantly affect the glucose metabolism, antihypertensive, lipid lowering agents, or hypouricemic agents and pregnant subjects were excluded. The study was approved from the Ethical review committee of Bangladesh Diabetic Association (BADAS). Ref no: BADAS-ERC/13/00106. Each participant gave written informed consent prior to study inclusion.
Anthropometric and clinical measurements
Standing height (cm) was measured using appropriate scales (Detect-Medic, Detect scales INC, USA) without shoes and was recorded to the nearest 5 mm. Weight (Kg) was measured by a balance machine was placed on a hard flat surface and checked for zero balance before measurement. The subjects were in the center of the platform wearing light cloths without shoes. Weight was recorded to the nearest 0.5 Kg. Body mass index (BMI) of the subjects was calculated using the formula of BMI = Weight (Kg)/ Height (m2). Waist circumference (WC) was measured to the nearest 0.5 cm with a soft non-elastic measuring tape. The WC was taken to the nearest standing horizontal circumference between the lower border of the 12th rib and the highest point of the iliac crest on the mid-axillary line at the end of normal expiration. Hip circumference (HC) was measured on the maximum circumference over the buttocks using soft non-elastic measuring tape and reading was taken to the nearest 0.5 cm. Participants were asked to breath normally, the reading were taken after gentle exhaling. The measuring tape was held firmly, ensuring its horizontal position. The tape was loose enough to allow the observer to place one figure between the tape and subject’s body. Waist to hip ratio (WHR) of the study subjects was calculated as the ratio of WC divided by HC. Body fat mass was measured by Omron Body Fat Monitor. Height in cm, weight in Kg, age in years and sex of subjects were set to the monitor. Then the subjects held the monitor by both hands with upper limbs horizontal in standing position. The machine was then put on and body fat mass (%) was recorded from the monitor. Blood pressure (BP) was measured using Barometric Sphygmomanometer and standard protocol was followed to record BP data. BP was measured in sitting position, with calf at the level of the heart. After 10 minutes of rest a second reading was taken and average was recorded. Recorded Korotkoff sound I (the first sound) and V (the disappearance of sound) denoted the systolic blood pressure (SBP) and diastolic blood pressure (DBP), respectively (according to WHO-IHS).
Biochemical analysis
Serum glucose, both at fasting and following ingestion of 75 g of glucose were measured by Glucose-Oxidase (GOD-PAP) method. Serum triglyceride (TG), serum cholesterol and serum high-density lipoprotein cholesterol (HDL-c) were measured by enzymatic colorimetric (GPOPAP) method. HbA1c was measured using the high-performance liquid chromatography (HPLC) method (Variant II, Bio-Rad Laboratories, Hercules, CA, USA). Liver enzymes like alanine transaminase (ALT) and gamma glutamyl transaminase (?-GT) and SUA were measured by standard enzymatic-colorimetric method (Randox, UK) using an automated analyzer (Hitachi 704, Tokyo, Japan). Low-density lipoprotein cholesterol (LDL-c) was calculated by the Friedewald equation. 15 Serum insulin was measured by an enzyme-linked immunosorbent (ELISA) method using commercially available kit (DRG-International, Germany). When specific performance characteristics were assessed for the measurement of SUA and insulin, the within and between run precision for SUA samples were 4.58% and 3.04% coefficient of variation (CV) and 6.3% and 4.25% respectively for insulin samples. Insulin secretory function (HOMA%B) and insulin sensitivity (HOMA%S) was calculated from fasting serum glucose and fasting serum insulin values by homeostasis model assessment (HOMA) using HOMA-CIGMA software. Insulin resistance was determined by the homoeostasis model assessment insulin resistance (HOMA-IR) and calculated according to the formula: Fasting serum insulin (µIU/ml) x fasting serum glucose (mmol/l)/22.5. 16
Radiological examinations
Abdominal ultrasounds were performed by a well trained physician using a 3.5 MHz linear transducer (Philips Ultrasound-Ay-MNT-15 TTK, HDI-4000, Netherland) in fasting state for grading the extent of fatty liver and to look for evidence of portal hypertension. The severity of echogenicity was graded as: Grade 0: normal echogenicity; Grade 1: slight, diffuse increase in fine echoes in liver parenchyma with normal visualization of diaphragm and intrahepatic vessel borders; Grade 2: moderate, diffuse increase in fine echoes with slightly impaired visualization of intrahepatic vessels and diaphragm; Grade 3: marked increase in fine echoes with poor or non-visualization of the intrahepatic vessel borders, diaphragm, and posterior right lobe of the liver. 17
Statistical analysis
Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) software package version 15.0 for Windows (SPSS Inc., Chicago, IL, USA). For the sociodemographic, clinical, anthropometric and biochemical characteristics, the normally distributed data with continuous variables were expressed as mean and standard deviation (SD), whereas the categorical variables were represented by frequency and percentage. Independent 2-sample t-tests were used to determine the mean ± SD. Sample size was calculated by using the regression model for individual predictors and it depends on the desired power (l-a), significance level (a), the number of predictors and the expected effect sizes. Sampling weights was used by using the formula of N > 50 + 8 m, where m is the number of independent variables (IVs) for testing the multiple correlation and N > 104 + m for testing individual predictors. In our study there was five IVs (table 3) and the calculated sample number was 50 + 8 (5) = 90 cases and 104 + 5 = 109 cases for testing individual predictors. These calculations were based on significance level of 5% (a = 0.05) and 80% power (p = 0.20). Binary logistic regression analysis was used to estimate the odds ratio (ORs) and 95% confidence interval (CI) for the presence of NAFLD. Bivariate Pearson’s correlation analysis was performed to see the correlation of SUA with significant variables of NAFLD. A multiple linear regression analysis was used to determine the effects of HOMA-IR on SUA concentrations in NAFLD subjects. The covariates used for adjustment were BMI, HbA1c and TG for continuous and dichotomous variables, respectively and they were identified primarily on the basis of published literature on the risk factors for diabetes. Since SUA has been considered as potential risk factor for the development of insulin resistance in NAFLD subjects and in order to get deeper understanding among their relationship, the study subjects were stratified in different HOMA-IR quartiles such as: Q1 < 1.5; Q2 1.5-2.0; Q3 2.1-2.8 and Q4 > 2.8 respectively. The HOMA-IR quartiles have been derived from the percentile distribution of insulin resistance among the study subjects. Distribution of SUA levels according to different quartiles of HOMA-IR in study subjects were compared using a one way analysis of variance (ANOVA). All statistical tests were two-sided and a p < 0.05 was recognized as the statistical significance level.
Results
Characteristics of the prediabetic subjects
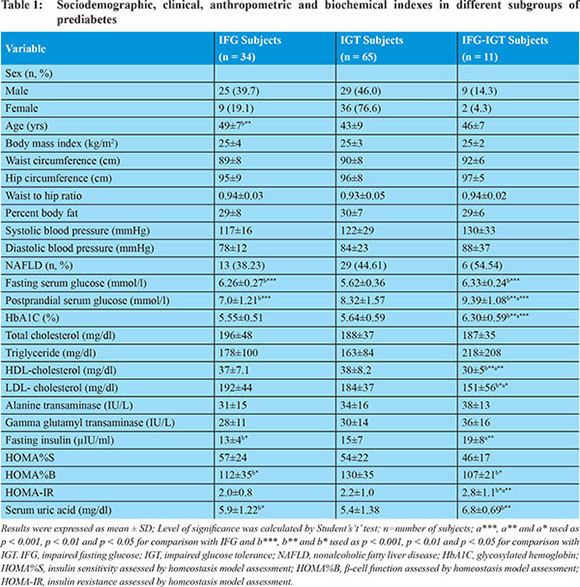
Among the total 110 prediabetic subjects, 30.9% (n = 34, M/F 25/9) was impaired fasting glucose (IFG), 59.1% (n = 65, M/F 29/36) was impaired glucose tolerant (IGT) subjects, and 10.0% (n = 11, M/F 9/2) was IFG-IGT respectively. Sociodemographic, clinical, anthropometric and biochemical characteristics of the study subjects are presented in Table 1. Age was significantly higher in IFG subjects (p = 0.003) compared to IGT subjects. Among glycemic profile, fasting serum glucose was significantly higher in IFG (p <0.001) and IFG-IGT subjects (p <0.001) as compared to IGT subjects and postprandial serum glucose was significantly higher in IFG-IGT subjects (p <0.001) as compared to IFG and IGT subjects whereas, in IGT subjects it was significantly higher compared to IFG subjects. HbA1C was significantly lower in IFG (p = 0.004) and IGT (p < 0.001) subjects compared to IFG-IGT subjects. Among lipidemic profile, HDL-cholesterol and LDL-cholesterol was significantly higher in IFG (p = 0.001 and p = 0.021) and IGT (p = 0.001 and p = 0.038) subjects as compared to IFG-IGT subjects. Among insulinemic profile, fasting serum insulin was significantly higher in IGT (p = 0.042) and IFG-IGT subjects (p = 0.002) as compared to IFG subjects. Insulin secretory capacity (HOMA%B) was significantly lower in IFG (p = 0.046) and IFG-IGT subjects (p = 0.038) compared to IGT subjects. Insulin resistance (HOMA-IR) was significantly lower in IFG (p = 0.022) and IGT subjects (p = 0.009) compared to IFG-IGT subjects. SUA was significantly higher in IFG (p = 0.047) and IFG-IGT subjects (p = 0.006) as compared to IGT subjects.
Characteristics of the prediabetic subjects according to their NAFLD status
Of the enrolled (n = 110) subjects, 56.4% (n = 62, M/F 35/27) was non NAFLD [IFG, 21 (33.9%); IGT, 34 (54.8%); and IFG-IGT, 7 (11.3%) respectively] and 43.6% (n = 48, M/F 28/20) was NAFLD [IFG, 13 (27.1%); IGT, 29 (60.4%); and IFG-IGT, 6 (12.5%) respectively]. Clinical, anthropometric and biochemical characteristics of the non NAFLD and NAFLD subjects are presented in Table 2. The NAFLD group had significantly higher waist and hip circumference, waist to hip ratio (WHR), percent body fat (%BF), blood pressure (SBP & DBP), HbA1c (glycosylated hemoglobin), total cholesterol (TC), triglyceride (TG), alanine transaminase (ALT), gamma glutamyl transaminase (?-GT), fasting serum insulin, SUA and HOMA-IR as well as lower levels of HDL-c, HOMA%S and HOMA%B respectively.
NAFLD subjects had significantly higher SUA and HOMA-IR levels
To get a deeper understanding of the relationship between SUA levels and HOMA-IR with NAFLD, all subjects were classified into quartiles by their HOMA-IR levels and are presented in Figure 1. The quartiles (Q) of HOMA-IR were Q1 < 1.5; Q2 1.5-2.0; Q3 2.1-2.8 and Q4 > 2.8 respectively. The HOMA-IR levels in Q1, Q2, Q3 and Q4 were significantly higher in NAFLD subjects compared to their non NAFLD subjects (Q1, 1.31 ± 0.13 vs 1.02 ± 0.19 p <0.001; Q2, 1.89 ± 0.10 vs 1.63 ± 0.14 p <0.001; Q3, 2.60 ± 0.17 vs 2.18 ± 0.13 p <0.001 and Q4, 4.08 ± 1.09 vs 2.93 ± 0.24 p <0.001 respectively). With increasing the levels of different quartiles of HOMA-IR the SUA also increased progressively and the increment is higher in NAFLD group compared to their non NAFLD counterparts.
Association of SUA and HOMA-IR with NAFLD after adjusting the effects of major confounding variable
Binary logistic regression analysis was done to explore the effects of SUA and HOMA-IR taking fatty liver group (non NAFLD considered as reference) as dependent variable adjusted with other confounding factors like BMI, HbA1c, and TG as independent variables. HbA1c [OR (95% CI): 3.505 (1.592-7.717) p = 0.002], SUA [OR (95% CI): 1.514 (1.058-2.167) p = 0.023] and HOMA-IR [OR (95% CI): 1.478 (1.042-2.096) p = 0.029] were found to be significant determinants of NAFLD where non NAFLD considered as reference after adjusting the effects of BMI and TG (Table 3).
Relationship of SUA with significant variables in the NAFLD subjects
Bivariate Pearson’s correlation analyses was performed for SUA with anthropometric, clinical and biochemical variables in the NAFLD subjects. SUA showed significant negative correlation with sex (r = -0.437, p = 0.002) and HDL-c (r = -0.458, p = 0.001). On the other hand, it showed significant positive correlation with BMI (r = 0.289, p = 0.046), SBP (r = 0.440, p = 0.002), HbA1c (r = 0.297, p = 0.037), TG (r = 0.288, p = 0.047), ?-GT (r = 0.590, p <0.001) and HOMA-IR (r = 0.288, p = 0.047) respectively (Table 4).
Association of SUA and HOMA-IR with NAFLD after adjusting major confounders
Multiple linear regression analysis was done among the NAFLD subjects using SUA as dependent variable and BMI, HbA1c, TG and HOMA-IR as independent variables. SUA showed significant positive association with TG (ß = 0.325, p = 0.033) and HOMA-IR (ß = 0.355, p = 0.027) among the NAFLD subjects after adjusting the effects of BMI and HbA1c (Table 5). As the sample size of our study was low we adjusted the only potential confounding variables of BMI, HbA1c and TG depending on their significant correlation with SUA in NAFLD subjects.
Discussion
Prediabetes and type 2 diabetes mellitus (T2DM) are believed to be associated with a worse metabolic profile in subjects with NAFLD which is a strong and independent risk factor for metabolic syndrome in the general adult population. 18 The exact etiology of NAFLD is not yet known, but hyperinsulinemia, insulin resistance and systemic inflammation are thought to play a major role in its pathogenesis. 19 Insulin resistance plays a central role in the vicious circle, which promotes lipolysis of the peripheral adipose tissue and increases the influx of free fatty acids into the liver. This insulin resistance leads to hyperinsulinemia, which increases the synthesis of serum uric acid (SUA) and decreases its renal excretion. 20 Our cross-sectional study revealed higher levels of SUA which is an important risk factor for the development of insulin resistance among NAFLD subjects suggesting a significant association between SUA and insulin resistance with NAFLD. Prediabetic subjects and persons with newly diagnosed diabetes have higher SUA levels than normoglycemic subjects 21 which plays plausible roles in the development of NAFLD. Pathophysiologic links between hyperuricemia, insulin resistance, and prediabetes have not been clearly established and are under investigation among NAFLD subjects. 22 Hyperuricemia is often the result of the under excretion of urate, and renal clearance of urate has been shown to be inversely related to the degree of insulin resistance. 23 SUA is related to increased renal glomerular pressure and increased renal sodium reabsorption, and these renal reactions are greatly enhanced by high insulin concentrations which may also affect renal tubular function and subsequent clearance of urate. 9,24 This raises the possibility that hyperuricemia may merely be a marker for the renal effect of hyperinsulinemia. The combined effects of insulin resistance and high SUA levels on renal functions may contribute to increased glucose intolerance, hypertension, diabetes and development of NAFLD.
In binary logistic regression analysis, we observed independent associations of HbA1c, SUA and HOMA-IR with the presence of NAFLD. These associations remained even after adjustment for other surrogate markers of NAFLD, such as BMI and TG ( Table 3). These findings indicate that hyperglycemia in prediabetes, which is induced by both insulin secretory defect and insulin resistance plays crucial role for increased levels of SUA by a decrease in urinary uric acid excretion 25 and accumulation of substrates for uric acid production. 26
From multiple linear regression analysis, we also observed a strong positive association of SUA with HOMA-IR in prediabetic subjects with NAFLD, independent of other potential confounders of BMI and HbA1c ( Table 5). Our data implied the crucial role of SUA in prediabetic subjects for the development of NAFLD, independent of markers of obesity. The significant association of SUA in the development of NAFLD due to HOMA-IR suggests their causal role in the pathogenesis of the disorder. The results of previous studies are consistent with our study. 27 Some researchers demonstrated that SUA levels are independent risk factors for NAFLD in healthy adults and recommended it as an additional measure in assessment of the risk for NAFLD. 28 One of the possible explanations for an association between SUA levels and NAFLD is that most prediabetic subjects with NAFLD also have insulin resistance. This effect of insulin resistance on SUA metabolism may partly explain elevated SUA levels in NAFLD subjects. Although SUA increase is also observed in individuals with insulin resistance, we found that the increased risks for NAFLD by hyperuricemia could not be explained merely through peripheral HOMA-IR. From our study we also found significantly higher levels of HOMA-IR at different quartiles with progressively higher SUA levels in NAFLD subjects. This results in accordance with Cai et al who found higher prevalence rate of NAFLD with the increase of SUA level but they did not see the relationship of different quartiles of HOMA-IR with progressive increases of SUA levels in NAFLD subjects. 29 The possible explanation is that SUA increase is individuals with insulin resistance, probably because hyperinsulinemia would cause lower renal UA excretion, 24,25 and indirect act on SUA via reduction of adipocyte sensitivity to insulin and then increases triglyceride lipolysis within adipose depots. 2
Limitations of the study: The diagnosis of NAFLD was based on ultrasonography and was not confirmed by liver biopsy, correlation between the different stages of NAFLD (by histologic picture) and the levels of different quartiles of SUA and insulin resistance could not be done, studies in larger prediabetes with different subgroups cohorts having NAFLD are needed to re-formulate the association of SUA with NAFLD and to explore whether these associations seemed to be mediated by insulin resistance. Fourth, the study needs to be completed involving adequate number of subjects to provide optimum statistical power.
From the present data it may concluded, that a high proportion (more than one-third) of the prediabetic subjects have NAFLD and the distribution of the disorder is almost similar in various subgroups of prediabetes. Insulin resistance which is a basic defect in prediabetes and NAFLD may also associates with elevated levels of circulating serum uric acid. The data also indicate that the hyperuricemic condition and NAFLD in prediabetic subjects occurs due to insulin resistance and their association is also affected by hyperglycemia and dyslipidemia among these subjects.
Conflict of Interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231.
- Choi SS, Diehl AM. Hepatic triglyceride synthesis and nonalcoholic fatty liver disease. Curr Opin Lipidol. 2008;19:295-300.
- Petta S, Camma C, Cabibi D, DiMarco V, Craxi A. Hyperuricemia is associated with histological liver damage in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2011;34:757-66.
- Gaffo AL, Edwards NL, Saag KG. Gout, Hyperuricemia and cardiovascular disease: how strong is the evidence for a causal link? Arthritis Res Ther. 2009;11:240.
- Powell EE, Jonsson JR, Clouston AD. Metabolic factors and non-alcoholic fatty liver disease as co-factors in other liver diseases. Dig Dis. 2010;28:186-191.
- Lonardo A, Loria P, Leonardi F, Borsatti A, Neri P, Pulvirenti M, et al. Fasting insulin and uric acid levels but not indices of iron metabolism are independent predictors of nonalcoholic fatty liver disease. A case-control study. Dig Liver Dis. 2002;34:204-11.
- Ryu S, Chang Y, Kim SG, Cho J, Guallar E. Serum uric acid levels predict incident nonalcoholic fatty liver disease in healthy Korean men. Metabolism. 2011;60:860-866.
- Lee S, Jin Kim Y, Yong Jeon T, Hoi Kim H, Woo Oh S, Park Y, et al. Obesity is the only independent factor associated with ultrasound-diagnosed non-alcoholic fatty liver disease: a crosssectional case-control study. Scand J Gastroenterol. 2006;41:566-572.
- Glantzounis GK, Tsimoyiannis EC, Kappas AM, Galaris DA. Uric acid and oxidative stress. Curr Pharm Design. 2005;11:4145-4151.
- Park SH, Kim BI, Yun JW, Kim JW, Park DI, Cho YK, et al. Insulin resistance and Creactive protein as independent risk factors for nonalcoholic fatty liver disease in nonobese Asian men. J Gastroenterol Hepatol. 2004;19:694-698.
- Hotamisligil GS. Inflammatory pathways and insulin action. Int J Obes Relat Metab Disord. 2003;27:S53-S55.
- Lv Q, Meng XF, He FF, Chen S, Su H, Xiong J, et al. High serum uric acid and increased risk of type 2 diabetes: a systemic review and meta-analysis of prospective cohort studies. PLoS One. 2013;8:e56864.
- Kodama S, Saito K, Yachi Y, Asumi M, Sugawara A, Totsuka K, et al. Association between serum uric acid and development of type 2 diabetes. Diabetes Care. 2009;32:1737-1742.
- World Health Organization. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia: Report of a WHO/IDF Consultation. Geneva: World Health Organization (2006).
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499-502.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-419.
- Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, et al. The utility of radiological imaging in non alcoholic fatty liver disease. Gastroenterology. 2002;123:745-50.
- Wang Y, Li YY, Nie YQ, Zhou YJ, Cao CY, Xu L. Association between metabolic syndrome and the development of non-alcoholic fatty liver disease. Exp Ther Med. 2013;6:77-84.
- Dowman JK, Tomlinson JW, Newsome PN. Pathogenesis of non-alcoholic fatty liver disease. QJM. 2010;103:71-83.
- Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S, et al. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: Sites and mechanisms. Diabetologia. 2005;48:634-642.
- Kramer CK, Mühlen DV, Jassal SK, Barrett-Connor E. Serum uric acid levels improve prediction of incident type 2 diabetes in individuals with impaired fasting glucose. The Rancho Bernardo Study. Diabetes Care. 2009;32:1272-1273.
- Nakanishi N, Okamoto M, Yoshida H, MatsuoY, Suzuki K, Tatara K. Serum uric acid and risk for development of hypertension and impaired fasting glucose or Type II diabetes in Japanese male office workers. Eur J of Epidem. 2003;18:523-530.
- Facchini F, Chen YD, Hollenbeck CB, Reaven GM. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA. 1991;266:3008-3011.
- Yamanaka H. Gout and hyperuricemia in young people. Curr Opin Rheumatol. 2011;23:156-160.
- Ter Maaten JC, Voorburg A, Heine RJ, Ter Wee PM, Donker AJ, Gans RO. Renal handling of urate and sodium during acute physiological hyperinsulinaemia in healthy subjects. Clin Sci (Lond). 1997;92:51-58.
- Galvan QA, Natali A, Baldi S, Frascerra S, Sanna G, Ciociaro D, et al. Effect of insulin on uric acid excretion in humans. Am J Physiol. 1995;268:E1-5.
- Jimba S, Nakagami T, Takahashi M, Wakamatsu T, Hirota Y, Iwamoto Y, et al. Prevalence of non-alcoholic fatty liver disease and its association with impaired glucose metabolism in Japanese adults. Diabet Med. 2005;22:1141-1145.
- Lee K. Relationship between uric acid and hepatic steatosis among Koreans. Diabetes Metab. 2009;35:447-51.
- Cai W, Song J, Zhang B, Sun Y, Yao H, Zhang Y. The prevalence of nonalcoholic fatty liver disease and relationship with serum uric acid level in Uyghur population. Sci World J. 2014;2014:1-7.
|
|
|
 |
|
|