48uep6bbphidcol2|ID
48uep6bbphidvals|2957
48uep6bbph|2000F98CTab_Articles|Fulltext
Median arcuate ligament syndrome (MALS) is a condition characterized by chronic, recurrent abdominal pain due to compression of the celiac artery by the median arcuate ligament of the diaphragm. The median arcuate ligament (MAL) is a tendinous ban spanning the right and left diaphragmatic crura anterior to the aorta. In MALS, the celiac artery is compressed by this fibrous band causing extrinsic compression anteriorly and thereby leading to partial or complete celiac artery occlusion. Based upon diagnostic studies using computed tomography (CT) of the abdomen or arteriography, the incidence of asymptomatic celiac artery stenosis appears to be approximately 7%. Few of these patients have hemodynamically significant stenosis that cause symptoms1. We present a case of a patient with MALS presenting with recurrent postprandial abdominal pain with occasional vomiting.
Case Report
A 12-year-old boy was admitted with symptoms of recurrent abdominal pain and intermittent vomiting of 4-months duration. Abdominal pain was non-colicky, upper abdominal in location and non-radiating; pain increased post prandially and was relieved by bowel rest. Associated occasional non-bilious vomiting aggravated by food intake was reported. There was no history of GI bleed, jaundice, weight loss, altered bowel habits or urinary complaints. Physical examination revealed tenderness over left hypochondrium but was otherwise normal.
Hematological and biochemical workup including blood counts, liver function tests, electrolytes, blood sugar level, amylase and lipase were normal. Electrocardiography and abdominal ultrasonography were normal. Upper GI endoscopy showed mild Helicobacter pylori negative gastritis and duodenal biopsy revealed mild nonspecific inflammation.
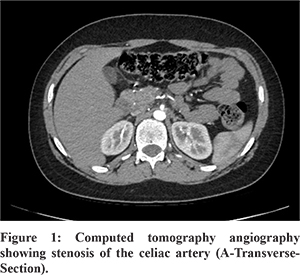
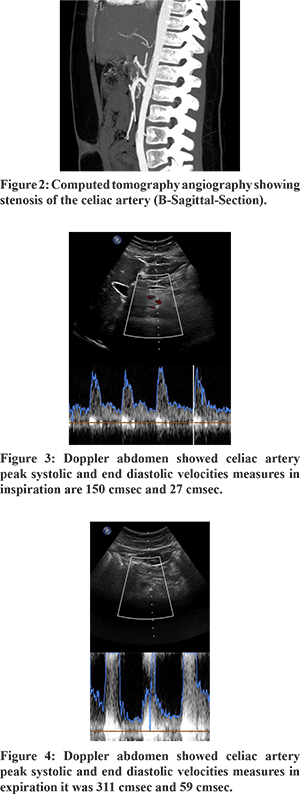
Subsequent evaluation was performed to identify the cause of pain. Barium Meal revealed normal esophageal and gastric motility and no abnormality. CT scan of the abdomen revealed tight stenosis of the celiac artery at its origin due to indentation by median arcuate ligament suggestive of MALS (Figure 1). The clinical significance of this finding was further confirmed by Doppler ultrasonography which revealed significant difference in the celiac artery peak systolic and end diastolic velocities during inspiration and expiration (150 cm/sec and 27 cm/sec and 311 cm/sec and 59 cm/sec respectively) (Figure 2). This finding confirmed the diagnosis of MALS. Other rare causes of abdominal pain like chronic lead poisoning, recurrent or autoimmune pancreatitis or porphyria were ruled out by relevant investigations.
Patient was subjected to laparoscopic division of the MAL. In a low lithotomy position,five intraabdominal ports were placed. Abdominal Aorta was identified and traced up to the coeliac axis and the coeliac artery and its branches were identified. The origin was seen to be compressed by a tight fibrous band of the MAL. This was divided completely so that the artery was free of any residual kinks. Patient had an uneventful postoperative course and was started orally after 48 hours and was discharged after 3 days.
At 10 days follow up, patient did not complain of abdominal pain or vomiting. At 6 months of telephonic follow up, patient remained asymptomatic.
Discussion
MALS is more prevalent in women compared with men (4:1), in age groups 30 and 50 years and those with a thin body habitus. This syndrome was first described in 1963 by Harjola1.
The postulated mechanisms of MALS are both ischemic and neuropathic. Compression of the celiac artery occurs due tothe abnormally low-lying ligament. Thiseffectis further aggravatedduring expirationas the diaphragm moves cranially, causing further compression of the celiac trunk.This compression leads to visceral ischemia and postprandial abdominal pain. Adjacent to the median arcuate ligament is the celiac plexus (or ganglion). This too may contribute to celiac artery compression. An acquired transient form of the syndrome has also been reported following blunt abdominal trauma and pancreatic surgical procedures, such as pancreaticoduodenectomy; likely related to the local trauma and resultant edema2.
Because of its rarity, MALS is low on the differential diagnosis for chronic abdominal pain. It may be suspected by a clinical triad of postprandial abdominal pain, weight loss, and sometimes an abdominal bruit3. A definitive diagnosis of MALS requires vascular imaging to confirm compression of the celiac artery by the median arcuate ligament (duplex ultrasound combined with advanced vascular imaging), preferably with respiratory maneuvers. During expiration, the diaphragm moves cranially, stretching the crura, and exacerbating the compression. Conversely, during inspiration, the diaphragm moves in a caudal direction and the crura become lax, relieving any compression4. Conventional angiography and / or recently multi-detector CT angiography is the gold standard for the diagnosis MALS. A sagittal view is particularly useful to detect the narrowing, which has acharacteristic hooked appearance and collateral vessels may also be noted3.
The key to successful outcomes is careful selection of patients for treatment. Asymptomatic patients with an incidental diagnosis do not require treatment. The goal of MALS treatment is to restore normal blood flow in the celiac axis. Classically, a simple surgical division of the fibrous ligament was performed. However, open surgery is more invasive and increases morbidity5. Recently, laparoscopic division of the MAL has proven to be equally effective as open surgery and with lower morbidity. Other case series of laparoscopic division of MAL show relief in nearly 80% patients. Typically, pain relief is immediate but it may take up to 6 weeks to resolve. Persistent symptoms have been successfully treated with angioplasty2. Other treatment options include open celiac artery revascularization-patch angioplasty, reimplantation of the celiac artery onto the aorta and aorto-celiac bypass5.
Conclusion
MALS is a rare cause of epigastric pain. Its diagnosis is challenging. MALS should be considered as differential diagnosis in patients with variable clinical features including postprandial epigastric pain, weight loss, diarrhea, nausea, and vomiting. Diagnosis can be confirmed by doppler ultrasonography and/or CT angiography. Laparoscopic surgical decompression is currently the treatment of choice in most patients.
References
- Kim EN, Lamb K, Relles D, et al. Median Arcuate Ligament Syndrome-Review of This Rare Disease. JAMA Surg 2016; 151:471.
- A-Cienfuegos J, Rotellar F, Valent´i V, et al. The celiac axis compression syndrome (CACS): critical review in the laparoscopic era. Rev EspEnferm Dig. 2010;102(3):193-201.
- Fong JK, Poh AC, Tan AG, Taneja R. Imaging findings and clinical features of abdominal vascular compression syndromes. AJR Am J Roentgenol 2014; 203:29.
- Erden A, Yurdakul M, Cumhur T. Marked increase in flow velocities during deep expiration: A duplex Doppler sign of celiac artery compression syndrome. Cardiovasc InterventRadiol 1999; 22:331.
- A. J. Duffy, L. Panait, D. Eisenberg, R. L. Bell, K. E. Roberts, and B. Sumpio, “Management of median arcuate ligament syndrome: a new paradigm,” Annals of Vascular Surgery, vol. 23, no. 6, pp. 778–784, 2009.