48uep6bbphidvals|167
48uep6bbphidcol4|ID
48uep6bbph|2000F98CTab_Articles|Fulltext
Since endoscopic insertion of a gastrostomy tube was firstdescribed in 1980 by Gauderer et al,[1] a multitude of commercialkits and variations of the percutaneous endoscopicgastrostomy (PEG) technique have been introduced, includingthe push (Sachs-Vine), pull (Ponsky), and introducer (Russell).PEG-tube insertion has been found to be a safe and effectiveprocedure replacing open gastrostomy for long-term enteralnutrition. A feeding gastrostomy tube can also be placed byinterventional radiologists under fluoroscopy or by surgeonsthrough surgery on the anterior abdominal wall. PEG-tubeoffers greater patient comfort, less frequent complications likedisplacement and greater improvement in the nutritionalstatus. It can remain functional for more than one year or longerand requires replacement through the same opening quiteinfrequently. The main reason for this advantage is the factthat this tube is made up of silicon material, an inert substancethat has neither local reaction nor any systemic complication.The most commonly performed are the push and pulltechniques, which have shown efficacy and safety in controlledtrials. Approximately 100,000 to 125,000 PEG procedures areperformed annually in the United States alone.[2] Despite theiroverall safety, a number of complications can occur following PEG placement.
Technique
The knowledge and adherence to the proper techniques ofPEG placement is crucial to avoid complications. The mostwidely used PEG technique is the “pull” method.[1]
There are several modifications of the original technique.The gastrostomy tube can be pushed rather than pulled intoplace by a “push” (Sacks-Vine) method.[3] In the “introducer”(Russell) method, the stomach is directly punctured and aFoley catheter placed over a guidewire.
Percutaneousgastrostomy has also been described without endoscopy,using a nasogastric tube for gastric insufflation, fluoroscopy,and a direct percutaneous catheter insertion.[4] The mostcommonly used method of placement is the pull technique.
After preparation of the abdomen, administration of prophylacticantibiotic and sedation/ analgesia, a complete upperendoscopy is performed. The stomach is insufflated, resultingin close apposition of the stomach to the abdominal wall. Apoint is chosen in the midepigastrium, where there is maximaltransillumination and indentation of the gastric lumen, withdirect pressure of a blunt pointer. A local anaesthetic is theninfiltrated into the area around the puncture site and a smallincision is made. A large-bore needle is inserted into the gastriclumen under endoscopic observation. A guidewire is threadedthrough the needle, grasped with endoscopic snare, and theneedle withdrawn. The endoscope-snare-guidewire iswithdrawn from the mouth as a single unit. The tapered end ofthe gastrostomy tube is then secured to the guidewire andpulled back down into the stomach, followed by endoscopicconfirmation of the internal bumper placement, which shouldbe snug against the gastric wall. An external bumper is usedto secure the PEG tube in place and prevent distal propagationof the internal bumper. The “push” (Sacks-Vine) method andthe “introducer” (Russell) method are the alternativetechniques of PEG tube placement. Procedural details of thesemethods are beyond the scope of this review.[3,5] The basicelements common to all PEG techniques are: (a) gastricinsufflation to bring the stomach into apposition with theabdominal wall; (b) percutaneous placement of a cannulainto the stomach; (c) passage of a suture or guidewire into thestomach; (d) placement of the gastomy tube; and (e)verification of the proper position.[1,3,4,5]
Indications of PEG
-
Head and neck cancers. PEG has become the mostacceptable and safest method for long term feedingsupport. It is useful particularly when surgery is extensiveand when combined with chemotherapy, radiotherapy orboth.
-
Malignant bowel obstruction including oesophagealcancer
-
Neurological conditions are the most common indicationsfor PEG and include:
-
Stroke (usually the most common indication for PEGand often vertebrobasilar strokes)
-
Disorders of swallowing
-
Multiple sclerosis
-
Neurosurgical disease
-
Parkinson’s disease
-
Brain tumours
-
HIV encephalopathy
-
Neonatal encephalopathy
-
Amyotrophic lateral sclerosis
-
Dementia (in which use is common but controversial)
-
Head injury patients
-
AIDS and HIV encephalopathy (improves nutritional statusbut not survival)
-
Crohn’s disease
-
Burns patients
Contraindications and patient selection
PEG insertion is safest with careful patient selection. PEGinsertion should be avoided in:
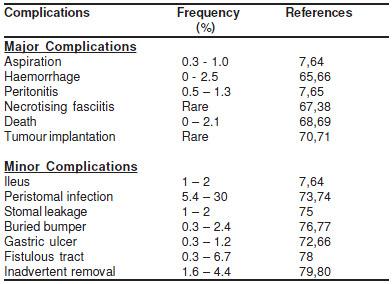
Incidence of complications (Table 1)
Several large prospective trials of PEG placement haveevaluated the efficacy and safety of the procedure. Thefrequency of complications observed in the various reportsdepends upon the definitions used and the population understudy. In one series, for example, complications weredescribed in 70% of 97 patients of which 88% were consideredminor, including tube dislodgement, peristomal woundleakage, and PEG wound infection.[6] A much lower rate ofcomplications was observed in another report of 314 patientsof whom 13% had minor and 3% had major complications,including gastric perforation, gastric bleeding, and hematomadevelopment.[7] Most studies have suggested thatcomplications are more likely to occur in elderly patients withco-morbid illnesses, particularly those with an infectiousprocess or who have a history of aspiration.[8]
Table 1 :Major and minor complications following PEGplacement
Minor complications
As mentioned above, complications from PEG tube placementmay be classified into major and minor. Minor complicationsinclude wound infection, leakage, and bleeding, cutaneous orgastric ulceration, pneumoperitoneum, temporary ileus, andgastric outlet obstruction.
Wound infection - Wound infection is more likely to occurwhen a PEG has been placed through a contaminatedprocedure field, or with poor technique in debilitated patientsand those who did not receive antibiotic prophylaxis.[9,10,11]
Most PEG wound infections will respond toa firstgeneration cephalosporin or a quinolones. Methicillin resistantStaphylococcus aureus have emerged as an important causeof PEG-site infections in some centres.[12]
At least two studies found that nasopharyngealdecontamination of patients with MRSA (in addition to standardprophylactic antibiotics) significantly reduced the incidence ofwound infections.[13,14] Another study observed thatadministration of a third generation cephalosporinintravenously and a povidone-iodine spray to the abdominalwall pre-PEG procedure reduced wound infections comparedto the intravenous cephalosporin administration or thepovidone-iodine spray used separately.[15] As per the latestreview in the Cochrane database, administration of systemicprophylactic antibiotics before PEG placement reducesperistomal infection.[16]
Fungal-related PEG infectious complications occur,although much less commonly than bacterial . These includefungal peristomal cellulitis, candidial peritonitis and intra-abdominal abscesses.[17,18,19,20]
Peristomal leakage — Peristomal leakage usually occurswithin the first few days following PEG placement. It is morelikely to occur in malnourished patients and those with diabeteswho may have poor tissue healing and are prone to woundbreakdown. In addition, placement of the external bolster ofthe PEG tube too tightly against the external abdominal wallmay lead to poor tissue blood flow, wound breakdown, andperistomal leakage.
Treatment should include correction of co-morbid factorssuch as malnutrition and elevated blood sugar, loosening ofthe external bolster, and local measures to address skinbreakdown (such as powdered absorbing agents or a skinprotectant such as a paste of zinc oxide). Placement of a largersized PEG tube through the same tract does not solve theproblem. Once the PEG tube tract has started to leak, placinglarger gastrostomy tubes through the same tract only servesto further distend and distort the tract and does not promotetissue growth or healing.
As a treatment method, the PEG tube can be removedoccasionally for 24 to 48 hours, permitting the tract to closeslightly; a replacement gastrostomy tube can then be placedthrough the same, partially closed tract.21 This technique workswell for patients whose PEG tube tract started to leak a monthor more after initial insertion. It does not work as well for patientswith early tract leakage since these patients usually experiencepoor wound healing from co-morbid disease processes.
In many patients with a mature PEG tract and peristomalleakage, the PEG tube will need to be fully removed, allowingthe tract to close completely. Another PEG tube can then beplaced at a different location on the abdominal wall. In ourexperience, the new PEG tube can be placed when there is atleast 50% closure of the old PEG tube tract, at which point the initiation of feeding will not have a significant impact on leakageor inhibition of tissue healing through the old PEG tube.
Pneumoperitoneum — Pneumoperitoneum is a commonpost-PEG placement complication.[22] It is thought to besecondary to the insufflation of air associated with theendoscopic procedure and needle puncture of the gastric wall.In the absence of peritonitis it has no consequences andshould not preclude feedings. However, pneumoperitoneummay cause confusion for clinicians in those patients whereclinical features raise concern about a ruptured viscous. Inthese settings, a contrast radiology study should be obtainedto confirm the position of the PEG tube within the stomach andto exclude a leak.
The presence of subcutaneous air has also beendescribed following PEG placement. It occurs from air beingintroduced between the cutaneous and subcutaneoustissues.[23] In the absence of other findings, it is inconsequentialand should not preclude feeding.[23]
Ileus — Some patients develop nausea and vomitingsubsequent to PEG placement, which may be due to transientgastroparesis. In rare patients, ileus develops, a complicationthat may be more likely in patients with significantpneumoperitoneum.[24] After gastric or duodenal perforation hasbeen excluded, patients who develop ileus should be treatedwith bowel rest and, if necessary, nasogastric decompression.These patients are identified by the presence of post-procedure abdominal distention, vomiting, and absence ofbowel sounds. Feeding should be withheld until the ileusresolves.
Bleeding — Hemorrhage following PEG tube placementis rare. There have been case reports of significant bleedingfollowing PEG including aortic perforation, gastric arteryperforation, and retroperitoneal hemorrhage.[25,26,27,28,29] Most bleedingmay be controlled by simple pressure over the abdominalwound. Appropriate measures should be taken to improveabnormal coagulation parameters. Gastric wall and rectussheath hematomas have been described.[28] These are usuallyself-contained lesions that do not require surgical intervention.
Bleeding occasionally develops in the PEG tube tract itself.In such cases, we suggest tightening the external bumperagainst the abdominal wall, thereby pulling the internalbumper against the gastric mucosa and compressing thePEG tube tract. Compression should be released within 48hours to avoid PEG tube tract wound breakdown. Only rarelywill surgical intervention be necessary for PEG associatedbleeding complications.
One case of severe upper gastrointestinal bleed wasreported following the use of T-fasteners to secure the stomachto the abdominal wall prior to PEG tube placement.[26] Six weeksafter PEG placement, a retained metal T-fastener was foundimbedded in the abdominal wall at the site of a major arterialbleed. Bleeding did not respond to endoscopic interventionand ultimately required surgery.
It is also necessary to attempt improvement of significantlyabnormal blood coagulation parameters prior to tractionremoval of PEG tubes to prevent tract hemorrhage. However,gastrostomy tube replacement devices with a balloon tip canbe placed percutaneously safely in patients with abnormalblood coagulation parameters unless it is anticipated that thegastrostomy tube tract will require dilatation prior to insertion.
Ulceration — In patients with longstanding PEG tubeplacement, an ulcer may develop underneath the internal tube bolster or on the gastric wall. This often responds to looseningof the external bolster, which allows the internal PEG tubebolster to be released from the gastric mucosa. In patientswho have a rigid internal bolster, the PEG should be exchangedfor one with a flexible internal bolster to reduce the potentialfor future gastric ulceration.
Ulceration of the contralateral gastric wall from the site ofthe gastrostomy tube can occur with balloon gastrostomyreplacement tubes. In some of these tubes, the tip of the PEGmay extend out from the inflated balloon and act as amechanical irritant. The balloon gastrostomy tube should beremoved and replaced with a non-balloon replacementgastrostomy tube or a replacement gastrostomy tube in whichthe tube tip is contained within the inflated balloon [30].
Clogging — One of the most common problems is tubedysfunction secondary to clogging from medications or enteralformulae.[31] All medications should be dissolved in water or anappropriate liquid substance or delivered in liquid form, ifavailable. A clinical pharmacist should be consulted if thereare questions. Bulking agents such as psyllium and resinssuch as cholestyramine should never be placed through thePEG tube. Patients and caregivers should be educated to theimportance of flushing water through the PEG tube after allmedication and enteral formula delivery. In the event of a PEGtube obstruction, flushing the tube with a 50 cc syringe isrecommended.
The best irrigant is warm water, which is superior to otherliquids such as juices or colas.[28] Pancreatic enzymes(dissolved in a bicarbonate solution and left to dwell within thePEG prior to water flushing) are also effective.[32] If this techniquefails, the gastrostomy tube can be cleared with a speciallydesigned gastrostomy tube declogging brush.
Tube dysfunction — As discussed above, one of the mostcommon causes of tube dysfunction is clogging frommedications or enteral formulae. Another common cause isdeterioration of the PEG tube. Deterioration is recognised onobserving pitting, ballooning, and a characteristic smell.Although this presents no real risk to the patient, the tube candevelop leaks and break, which makes tube feeding difficultor impossible. Microscopic examinations have demonstratedthat tube deterioration is caused by yeast implantation into thewall of the tube.[33] A randomised controlled trial suggested thatdeterioration leading to tube dysfunction was significantly morecommon with silicone compared to polyurethane PEG tubes.[34]
No preventive measures have been established as effectivefor preventing this problem. In my own practice, I recommendflushing the tube daily with 3 to 5 cc of ethanol in an attempt to“sterilise” the tube lumen. There is no absolute time periodwithin which the PEG tube should be removed and exchangedto prevent tube dysfunction. The standard of care is to permitthe tubes to remain in place until some tube dysfunction, suchas clogging or deterioration, prevents adequate feedings ormedication.
Another aspect of gastrostomy tube failure is the earlydeflation of the balloon, which serves as the internal bolster.This is usually encountered with the use of balloongastrostomy replacement tubes. There are no prospectivedata comparing one manufacturer’s gastrostomy tube balloonto another manufacturer’s gastrostomy tube balloon.Evaluation of PEG tube materials suggest that balloonsconstructed of polyurethane may be more durable thanballoons constructed of silicone.35 There are commercially available gastrostomy replacement tube devices where aflexible bolster, rather than a balloon, serves as the internalbolster. The internal bolster is distended with a rigid stylet andpassed through the PEG ostomy site. This resolves theballoon deflation issue. However, the stylet can cause damageto the existing PEG tube tract on insertion if not placed properly.
Gastric outlet obstruction — PEG tubes can migrateforward into the duodenum and cause gastric outletobstruction.[36] This occurs if the external bolster on the PEGtube is allowed to migrate away from the abdominal wall,allowing the PEG tube to slide forward through the gastrostomytract and into the duodenum. A similar problem has beenreported with balloon gastrostomy tubes, where the inflatedballoon is allowed to migrate through the pylorus resulting inan obstruction.[37] This complication can be avoided by makingsure the external bolster remains at the same centimetre markon the gastrostomy tube after initial proper positioning.
Major complications
Major complications include necrotising fasciitis, esophagealperforation, gastric perforation, colocutaneous fistula, buriedbumper syndrome, and inadvertent PEG removal.
Necrotising fasciitis — Necrotising fasciitis (necrosis ofthe fascia layers) is a rare complication of PEG placement.[38,39]Patients with diabetes, wound infections, malnutrition, and apoor immune system are at increased risk.
Traction and pressure on the PEG wound may predisposeto the development of necrotising fasciitis. One studydemonstrated that patients who had their PEG tube externalbolster set directly against the abdominal wall were more likelyto develop wound infection, peristomal drainage, and fasciitiscompared to patients whose external PEG bolster was left 3cm from the abdominal wall.[40] It was hypothesised that thedistant placement of the external bumper preventedcompression of the tissue in the PEG tube tract and woundbreakdown. This hypothesis was confirmed in a study on dogsin which gastric mucosal histology showed severeinflammation when the PEG tube external bolsters were placeddirectly against the abdominal wall as compared to externalbolsters that were left 4 cm from the abdominal wall.[41]
Prevention of necrotising fasciitis is imperative sincetreatment requires large surgical debridement, antibiotics, andextensive hospital support. It is important to allow the externalbolster of the PEG tube to “free-float” 1 cm to 2 cm from theabdominal wall after PEG placement to prevent thiscomplication. Loose apposition of the stomach to theabdominal wall does not result in peritoneal leakage since anearly PEG tube tract forms as a result of tissue oedema andassociated tissue secretions.
It is also important to make at least a 1 cm skin incisionprior to PEG placement to avoid creating too tight a PEG tubetract wound once the PEG tube is pulled through the woundfollowing PEG placement. However, there is controversyinvolving this practice. A controlled trial randomly assigned 50patients to PEG placement with and without abdominal wallincision.[42] There was no difference in wound healing betweenthe two groups at seven days. Twelve percent of the noabdominal wall incision group ultimately required anabdominal wall incision for the PEG tube procedure to becompleted.
Wound care is important following PEG tube placement.As with any other surgical procedure, lesser manipulation isencouraged. There are no prospective evaluations supportingthe use of topical antibiotics as a preventative measure forwound infection following PEG placement. It is our practice tosimply clean the wound with full strength hydrogen peroxideand cover it with gauze dressing. The gauze dressing ischanged and the PEG wound is cleansed with hydrogenperoxide daily for seven days. Following this, the wound canbe cleaned with simple soap and water. The gauze dressingcan be eliminated unless there is leakage around the PEGtube that is soiling a patient’s clothes.
Gastric and esophageal perforations with upperendoscopy are known, but rare complications. There are noappropriate reports from which to derive estimates of howfrequently these occur.
Buried bumper syndrome — Buried bumper syndromeis a long-term consequence of tight apposition of the externalbolster of the PEG tube against the abdominal wall.[43] Theinternal bolster of the PEG tube slowly erodes into the gastricwall as tension is created on the PEG tube tract, whichultimately causes pain and the inability to administer feeds.The diagnosis can be confirmed on endoscopy, whichdemonstrates the internal bumper buried within the gastricmucosa.
Treatment of the buried bumper syndrome depends onthe type of PEG tube used.[44] If the internal bolster is collapsible,as it is on externally removable PEG tubes, the PEG tube canbe removed by simple external traction. In a modification ofthis technique, the buried bumper PEG tube can be cut shortand a guide-wire passed through the stump into the gastriccavity.[45] The guide-wire is snared and pulled out of the oralcavity and attached to a new PEG. The guide-wire at theabdominal surface is pulled, dragging the new PEG into thegastric cavity. The dilating portion of the new PEG engages theburied bumper on the old PEG. As the new PEG is pulledthrough the abdominal wall, the old PEG is pushed out of theabdominal wall and removed.
In contrast, if the internal bumper on the PEG tube is rigid,as it is on endoscopic only removable PEG tubes, the PEGtube may have to be removed by PEG wound tract cut-down orthe push-pull T-technique. The push-pull T-technique requiresthe PEG tube to be cut 3 cm from the abdominal wall. Thepatient undergoes endoscopy and a snare is passed throughthe PEG tube opening in the gastric wall to the outside throughthe PEG tube. An additional short piece of PEG tube is cut fromthe excess PEG tubing. The snare is opened and this shortpiece of tubing is grasped and pulled back against the PEGtube creating a T-shape. A Kelly clamp is placed across the T-shape. The endoscopist slowly removes the endoscope,snare, and PEG tube orally as a second operator pushes theKelly clamp and PEG tube into the gastric lumen. Thiscombined procedure frees the internal bumper from the gastricwall. Once the PEG tube is removed, a new PEG tube can bereplaced through the existing PEG tract using directendoscopic visualisation. A standard PEG tube placementtechnique should be used to permit the PEG tube dilator to re-expand the partially closed PEG tube tract.
Prevention of the buried bumper syndrome requires goodnursing care and patient instruction. As mentioned above, theexternal bolster of the PEG tube should be left 1 to 2 cm from the abdominal wall. Gauze pads should be placed over theexternal bolster, not underneath, which would create pressureon the PEG tube tract. In addition, the gastrostomy tube itselfshould be pushed forward into the wound slightly and rotatedduring daily nursing care. This will ensure that the internalbumper does not become buried in the gastric mucosa. Afterrotation, the PEG should be replaced into its original position.
Colo-cutaneous fistula — A colo-cutaneous fistula is a rarecomplication associated with PEG placement.47 It occurs as aresult of interposition of bowel, usually the splenic flexure,between the anterior abdominal wall and the gastric wall. ThePEG tube is placed directly through the bowel into the stomach.
Patients in whom this complication has occurred are oftenasymptomatic, except for transient fever or ileus. The problemis usually discovered months after initial PEG tube placementwhen the original PEG tube is removed for gastrostomy tubereplacement. As the replacement gastrostomy tube is passedblindly at the bedside, it is pushed through the PEG tractopening in the abdominal wall and into the colon, but cannotfind its way back into the stomach. Once the tube feedings arerestarted, the patient develops diarrhoea from colonic tubefeedings and dehydration from not receiving fluids or nutrition.
This complication can often be treated by removing thePEG tube, allowing the fistula to close.[46] However, surgery issometimes necessary to correct the internal gastric-bowelfistula.
Prevention of this complication is related to the initial PEGtube procedure. Relying on a combination of trans-illuminationand finger palpation of the abdominal wall in choosing anappropriate PEG tube site rather than one of these techniquesalone will assure a safe PEG tube entrance site. In questionablesituations, an 18 or 22 gauge needle should be passedthrough the PEG tube site prior to PEG tube placement. Theneedle should be withdrawn slowly with an attached syringecreating back pressure. The presence of a sudden bolus ofair or stool within the syringe suggests passage through thebowel. However, this technique has not been subjected toprospective evaluation. In questionable situations where safeaccess site cannot be determined, ultrasound or CAT scanguidance can assist in delineating a safe location.
Inadvertent PEG tube removal — Inadvertent PEG tuberemoval is a common complication usually occurring incombative or confused patients who pull on the tube. ManyPEG tubes today are designed to be externally removed with10 to 14 pounds of external pull pressure.
PEG tubes that are inadvertently removed within the firstfour weeks of PEG tube placement should not be replacedblindly at the bedside. Because the PEG tube tract may nothave matured adequately, the gastric wall and the abdominalwall may have separated, leaving a rent in the gastric wall.Thus, blind replacement of the PEG tube at the bedside mayresult in its placement in the peritoneal cavity.
If a patient has had their PEG tube removed early on (priorto four weeks after initial placement) the patient canimmediately be brought back to the endoscopy suite for repeatPEG tube placement through the same PEG tube site.[48]Patients should be treated with intravenous antibiotics, andmonitored for signs of peritonitis, which would require surgicalintervention. If there is any concern about the possibility of areplacement gastrostomy tube being positioned into theperitoneal cavity, a water soluble contrast study through thegastrostomy tube should be obtained to confirm properposition prior to the initiation of feedings.
Other intra-abdominal complications — A variety of intra-abdominal complications have been described in casereports. Although rare, they must be recognised by theinterventional endoscopist.
-
Small bowel obstruction from a small bowel wallhematoma following PEG placement.[
49] The hematomawas on a jejunal loop of bowel near the stomach. Anoperative procedure allowed evacuation of the hematomaand resolution of the small bowel obstruction.
-
Intrahepatic placement of a PEG tube.[
50] The originallyinserted PEG tube malfunctioned and was replaced with aballoon gastrostomy tube two and a-half years afterplacement. It was difficult to push the replacement tubeback through the PEG fistula site. A contrast study showedthat the balloon gastrostomy tube was inflated within theliver. Contrast from the tube entered the portal venoussystem. A fistula tract had developed between the liver andthe stomach. Subsequent surgical exploration allowed thetube to be removed safely with resection of the gastro-hepatic fistula tract, argon gas plasma coagulation of theliver bed to prevent bleeding and replacement of the PEGwith a Stamm gastrostomy.
-
Herniation of the stomach through a PEG tube fistula site.[
51]The patient had a leaking PEG site one year after insertion.A bulge was noted at the PEG tube site on the abdominalwall when the patient coughed. A CAT scan demonstratedthat a portion of the stomach had herniated through thePEG site. The PEG was removed, but the PEG fistularemained open. Surgical repair of the fistula wassuggested. However, the patient died of aspirationpneumonia prior to definitive surgical therapy.
-
Abdominal wall pain can occur and persist after PEGplacement. The patient work-up should include a fullexamination to rule out infection of the abdominal wall.This may include a CT scan to rule out abdominal wallabscess. In some cases the pain will be consistent withneuropathic pain, in which case the remedy is often removalof the PEG and insertion at a different site. Abdominal wallinjection with an anesthetic agent may also be helpful.
-
Peritonitis has been reported from leakage of gastriccontents from the gastrostomy site into the peritoneal cavitywith the PEG tube in situ.[
52] In addition, infusion of a tubefeeding formula can lead to a combination of a chemicaland bacterial peritonitis.[
8] It has been hypothesised thatperitonitis develops when the introducer needle enters thestomach tangentially rather than directly through theabdominal wall, leading to a long laceration along thegreater curvature which allows for escape of gastriccontents.
-
PEG tract tumour seeding — Patients with proximal GItract cancers, such as head and neck and esophagealcancers are at risk of tumor seeding from the tumour siteto the PEG tube tract by mechanical transfer.[
53,
54] The PEGtube can facilitate transfer of tumour cells whilst it is adjustedinto its final position across the gastric and abdominalwalls. The use of an over tube across the proximalgastrointestinal tract tumour site should allow the PEGtube to be placed through the over tube without the risk ofPEG tube tract seeding. There are currently no prospectivestudies comparing over tube versus no over tube PEGplacement in patients with proximal GI tract cancersas a mechanism for preventing PEG tube tract tumourseeding.
PEG versus surgical gastrostomy
Studies comparing surgical gastrostomy to PEG have shownno difference in morbidity or mortality.[55] However, PEGs areless expensive and save time. Thus, it seems prudent toreserve surgical gastrostomy for patients who are alreadygoing to the operating room for another surgical procedure orfor patients in whom an endoscopy cannot be performed orwhere an anatomical aberration prevents a safe percutaneousapproach for PEG placement.
An alternative to surgery in patients in whom a PEG cannotbe placed for anatomical reasons is a combination endoscopicand radiologic approach in which a CT scan or ultrasound isused to determine a safe site to access the stomach.[56] Aninterventional radiologist can also place a gastrostomy tubeusing similar radiographic assistance to locate a safe accesssite.
Early versus delayed feeding after PEG
PEG feedings have traditionally been delayed for several hoursto overnight after PEG placement because of concern thatearlier feeding would increase the risk of peritoneal leakageor aspiration. However, several studies have suggested thatearly feeding (>4 hours after PEG placement) may be as safeas feeding later on.[57] A meta-analysis of six studies thatcompared early versus delayed or next day feeding (with atotal of 467 patients) found no statistically significantdifferences in patient complications or death.[57] By contrast, astatistically significant increase in gastric residual volumesduring day one was noted in the early group, the clinicalconsequences of which were unclear.
However, the relatively small number of patients studiedmakes the benefits of this practice ambiguous. This wasreflected in the 95% confidence interval for the risk of deathwithin 72 hours in the above meta-analysis, which rangedfrom an 80% decrease in the risk with early feeding to asmuch as a 75% increase in the risk. Thus, more studies areneeded before such a practice can be confidently adopted. Atour institution, we begin with administering water andmedications through the PEG tube on the same day as PEGplacement, often four hours post-procedure. Tube feedingsare initiated the following day.
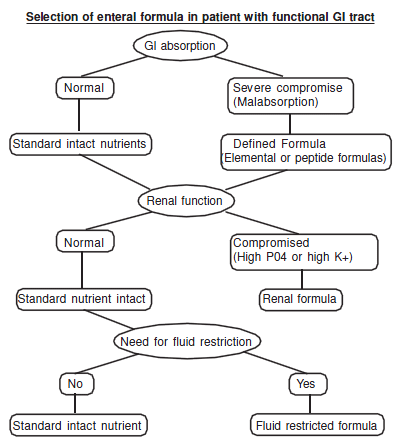
Feeding protocol
Rate of Administration
Adults:
A. Gastric feeding
a. Standard formulae should be initiated at a rate of 50 cc/hr unless there is significant concern regarding gastricmotility
b. If tolerated the rate of feeding can be increased by 25cc/hr every 4-8 hours until the goal is met.
c. Elemental formulae should be administered at fullstrength at 25cc/hr for the first 12 hours then improvedby 25 cc/hr every 6-12 hours until the goal is met
d. 2 Kcal/cc formulae should be instituted at 25 cc/hr andadvanced as elemental formulae even if the patienthas been on a standard formula prior to this formulachange.
Children:
A.Gastric feeds (PEG):
1.Initiation:
Infant <1: 30 mL q 3hr x 2
Toddler 1-5: 50 mL q 3hr x 2
Child > 5: 75 mL q 3hr x 2
Adolescent 12-14 100 mL q 3hr x 2
2.If bolus feeds tolerated then begin continuous feedand then advance as tolerated:
Infant <1: 10 mL/hr x 24 hrs
Toddler 1-5: 20mL/hr x 24 hrs
Children >5: 30mL/hr x 24 hrs
3. Gastric residual evaluation: Check residual q 4 hrs.
a. Bolus feeds: If residual volume is more than halfthe volume of the last bolus, withhold feeds
b. Continuous Feedings: If residual volume is morethan twice the hourly rate consult the doctor.
Formula selection
Special settings
A number of settings may be encountered in which PEGplacement is riskier, or that require modification of the standardtechnique.
Prior abdominal surgery — Patients who have had priorabdominal surgery can undergo placement of a PEG. However,extra care needs to be taken to avoid passing the tube throughinterpositioned bowel.[58] In these settings, PEG placementshould never be performed without confirming a safe accesssite by both finger palpation and transillumination.
Obesity — It may be difficult to transilluminate theabdominal wall in patients who are obese or have a thick abdominal wall. In such patients, adequate percutaneousaccess site can usually be palpated. A larger bedside incisioncan be made, and the fat tissue spread until the anterior rectusfascia is reached, after which a standard PEG tube can beplaced using conventional technique.[59] The external woundshould be closed with sutures or clips.
Use of a spinal needle (9 cm long) as the introducer needlehas been described in patients who are markedly obese (BMI>40 kg/m2).[60] In most instances abdominal walltransillumination could not be obtained, but finger palpationwas seen on the gastric mucosa with the endoscope. As thespinal needle was advanced, continuous aspiration on theneedle was maintained to monitor for entry into the colon orsmall intestine. A .025 cm guide-wire was required to fit throughthe spinal needle. This technique has been reported withsuccess and without complications in six patients with a BMI>60 kg/m2[61]
Pregnancy — Case reports have demonstrated safepercutaneous administration in women as advanced as 26weeks of pregnancy [62]. The risk of conscious sedation mustbe weighed against the need for nutritional support in thesepatients. An anaesthesia consultant can assist in safesedation.
Ascites — The presence of ascites is often acontraindication to PEG placement because of the fear ofabdominal fluid leakage and peritonitis. However, case reportshave demonstrated that large volume paracentesis before andfor the first week after PEG placement combined with the useof broad spectrum antibiotics has been associated withimproved patient outcome.[63] There have been no prospectivetrials confirming the safety of this technique.
Summary and recommendations
-
Despite their overall safety, a number of complications canoccur following PEG placement. Most studies havesuggested that complications are more likely to occur inelderly patients with co-morbid illness, particularly thosewith an infectious process or who have a history ofaspiration.
-
Minor complications include wound infection, woundleakage, wound bleeding, cutaneous or gastric ulceration,pneumoperitoneum, temporary ileus, and gastric outletobstruction.
-
Major complications include necrotising fasciitis,esophageal perforation, gastric perforation, majorgastrointestinal bleeding, colo-cutaneous fistula, buriedbumper syndrome, and inadvertent PEG removal.
-
A number of settings may be encountered in whichplacement of a PEG is riskier, or that require modificationof the standard technique.
-
A variety of intra-abdominal complications have beendescribed in case reports. Although rare, they must berecognised by the interventional endoscopist.
References
-
Gauderer MW, Ponsky JL, Izant RJ Jr. Gastrostomy withoutlaparotomy: A percutaneous, endoscopic technique.
J PediatrSurg. 1980;15:872–5.
-
Duszak R Jr, Mabry MR. National trends in gastrointestinal accessprocedures: an analysis of Medicare services provided byradiologists and other specialists.
J Vasc Interv Radiol.2003;14:1031–6.
-
Hogan RB, DeMarco DC, Hamilton JK, Walker CO, Polter DE.Percutaneous endoscopic gastrostomy – to push or pull. Aprospective randomized trial.
Gastrointest Endosc1986;32:253–8.
-
Willis JS, Oglesby JT. Percutaneous gastrostomy: furtherexperience.
Radiology 1985;154:71–4.
-
Russell TR, Brotman M, Norris F. Percutaneous gastrostomy. Anew simplified and cost-effective technique.
Am J Surg.1984;148:132–7.
-
Taylor CA, Larson DE, Ballard DJ, Bergstrom LR, Silverstein MD,Zinsmeister AR, et al. Predictors of outcome after percutaneousgastrostomy: A community-based study.
Mayo Clin Proc.1992;67:1042–9.
-
Larson DE, Burton DO, Schroeder KW. Percutaneous endoscopicgastrostomy: Indications, success, complications and mortality in314 consecutive patients.
Gastroenterology 1987;93:48–52.
-
Raha SK, Woodhouse K. The use of percutaneous endoscopicgastrostomy (PEG) in 161 consecutive elderly patients.
Age Ageing1994;23:162–3.
-
Sharma VK, Howden CW. Meta-analysis of randomized, controlledtrials of antibiotic prophylaxis before percutaneous endoscopicgastrostomy.
Am J Gastroenterol. 2000;95:3133–6.
-
Ahmad I, Mouncher A, Abdoolah A, Stenson R, Wright J, Daniels A,et al. Antibiotic prophylaxis for percutaneous endoscopicgastrostomy—a prospective, randomized, double-blind trial.
Aliment Pharmacol Ther. 2003;18:947–8.
-
Jafri NS, Mahid SS, Minor KS, Idstein SR, Hornung CA, GalandiukS. Meta-analysis: antibiotic prophylaxis to prevent peristomalinfection following percutaneous endoscopic gastrostomy.
Aliment Pharmacol Ther. 2007;25:647–56.
-
Chaudhary KA, Smith OJ, Cuddy PG, Clarkston WK. PEG siteinfections: The emergence of methicillin resistant Staphylococcusaureus as a major pathogen.
Am J Gastroenterol.2002;97:1713–6.
-
Horiuchi A, Nakayama Y, Kajiyama M, Fujii H, Tanaka N.Nasopharyngeal decolonization of methicillin-resistantstaphylococcus aureus can reduce PEG peristomal woundinfection.
Am J Gastroenterol. 2006;101:274–7.
-
Thomas S, Cantrill S, Waghorn DJ, McIntyre A. The role of screeningand antibiotic prophylaxis in the prevention of percutaneousgastrostomy site infection caused by methicillin-resistantStaphylococcus aureus.
Aliment Pharmacol Ther.2007;25:593–7.
-
Radhakrishnan NV, Shenoy AH, Cartmill I, Sharma RK, George R,Foster DN, et al. Addition of local antiseptic spray to parenteralantibiotic regimen reduces the incidence of stomal infectionfollowing percutaneous endoscopic gastrostomy: A randomizedcontrolled trial.
Eur J Gastroenterol Hepatol. 2006;18:1279–84.
-
Lipp A, Lusardi G. Systemic antimicrobial prophylaxis forpercutaneous endoscopic gastrostomy.
Cochrane Database SystRev. 2006;:CD005571.
-
Patel AS, DeRidder PH, Alexander TJ, Veneri RJ, Lauter CB. Candidacellulitis: a complication of percutaneous endoscopic gastrostomy.
Gastrointest Endosc. 1989;35:571–2.
-
Murugasu B, Conley SB, Lemire JM, Portman RJ. Fungal peritonitisin children treated with peritoneal dialysis and gastrostomy feeding.
Pediatr Nephrol. 1991;5:620–1.
-
Bell SC, Elborn JS, Campbell IA, Shale DJ. Candida albicans infectioncomplicating percutaneous gastrostomy in cystic fibrosis.
Br JClin Pract. 1995;49:109–10.
-
Alkartha A, Kawji AS, Alder DG. First reported case of Candidagalbrata perihepatic abscess as a complication of percutneousendoscopic gastrostomy tube placement.
J Clin Gastro.2007;41:335
-
Tsang TK, Eaton D, Falconio MA. Percutaneous ostomy dilation: atechnique for dilating the closed percutaneous endoscopicgastrostomy sites and reinserting gastrostomies.
GastrointestEndosc. 1989;35:336–7.
-
Wiesen AJ, Sideridis K, Fernandes A, Hines J, Indaram A, WeinsteinL, et al. True incidence and clinical significance ofpneumoperitoneum after PEG placement: a prospective study.
Gastrointest Endosc. 2006;64:886–9.
-
Stathopoulos G, Rudberg MA, Harig JM. Subcutaneous emphysemafollowing PEG.
Gastrointest Endosc. 1991;37:374–6.
-
Dulabon GR, Abrams JE, Rutherford EJ. The incidence andsignificance of free air after percutaneous endoscopicgastrostomy.
Am Surg. 2002;68:590–3.
-
Lau G, Lai SH. Fatal retroperitoneal haemorrhage: an unusualcomplication of percutaneous endoscopic gastrostomy.
ForensicSci Int. 2001;116:69–75.
-
Schurink CA, Tuynman H, Scholten P, Arjaans W, Klinkenberg-Knol EC, Meuwissen SG, et al. Percutaneous endoscopicgastrostomy: complications and suggestions to avoid them.
Eur JGastroenterol Hepatol. 2001;13:819–23.
-
Chong C, Derigo L, Brown D. Massive gastric bleeding: a rarelyseen subacute complication of percutaneous endoscopicgastrostomy.
Intern Med J. 2007;37:787–8.
-
Ubogu EE, Zaidat OO. Rectus sheath hematoma complicatingpercutaneous endoscopic gastrostomy.
Surg Laparosc EndoscPercutan Tech. 2002;12:430–2.
-
Mackenzie SH, Fang JC, Kuwada SK. Severe upper-GI bleeding ina patient with PEG tube placement by the radiologic push method.
Gastrointest Endosc 2007;65:935–7.
-
Kazi S, Gunasekaran TS, Berman JH, Kavin H, Kraut JR. Gastricmucosal injuries in children from inflatable low-profile gastrostomytubes.
J Pediatr Gastroenterol Nutr 1997;24:75–8.
-
Metheny N, Eisenberg P, McSweeney M. Effect of feeding tubeproperties and three irrigants on clogging rates.
Nurs Res.1988;37:165–9.
-
Sriram K, Jayanthi V, Lakshmi RG, George VS. Prophylactic lockingof enteral feeding tubes with pancreatic enzymes.
JPEN J ParenterEnteral Nutr. 1997;21:353–6.
-
Iber FL, Livak A, Patel M. Importance of fungus colonization infailure of silicone rubber percutaneous gastrostomy tubes (PEGs).
Dig Dis Sci. 1996;41:226–35.
-
Blacka J, Donoghue J, Sutherland M, Martincich I, Mitten-Lewis S,Morris P, Dwell time and functional failure in percutaneousendoscopic gastrostomy tubes: a prospective randomized-controlled comparison between silicon polymer and polyurethanepercutaneous endoscopic gastrostomy tubes.
Aliment PharmacolTher. 2004;20:875.
-
DeLegge RL, DeLegge MH. Percutaneous endoscopic gastrostomyevaluation of device materials: are we “failsafe”?
Nutr Clin Pract.2005;20:613–7.
-
Fischer LS, Bonello JC, Greenberg E. Gastrostomy tube migrationand gastric outlet obstruction following percutaneous endoscopicgastrostomy.
Gastrointest Endosc. 1987;33:381–2.
-
Chong VH. Gastric outlet obstruction caused by gastrostomy tubeballoon.
Indian J Gastroenterol. 2004;23:80.
-
Cave DR, Robinson WR, Brotschi EA. Necrotizing fasciitis followingpercutaneous endoscopic gastrostomy.
Gastrointest Endosc.1986;32:294.
-
Martindale R, Witte M, Hodges G, Kelley J, Harris S, Andersen C.Necrotizing fasciitis as a complication of percutaneous endoscopicgastrostomy.
JPEN J Parenter Enteral Nutr. 1987;11:583–7
-
Chung RS, Schertzer M. Pathogenesis of complications ofpercutaneous endoscopic gastrostomy.
Am Surg.1990;56:134–7.
-
DeLegge MH, Lantz G, Kazacos . Effects of external bolster tensionon PEG tube tract formation (abstract).
Gastrointest Endosc1996;43:349.
-
Sedlack, RE, Pochron, NL, Baron, TH. Percutaneous endoscopicgastrostomy placement without skin incision: results of arandomized trial.
JPEN J Parenter Enteral Nutr. 2006;30:240–5.
-
Klein, S, Heare, BR, Soloway, RD. The “buried bumper syndrome”:A complication of percutaneous endoscopic gastrostomy.
Am JGastroenterol 1990;85:448–51.
-
Venu, RP, Brown, RD, Pastika, BJ, Erikson LW Jr. The buried bumpersyndrome: A simple management approach in two patients.
Gastrointest Endosc. 2002;56:582–4.
-
Boyd JW, DeLegge MH, Shamburek RD, Kirby DF. The buried bumpersyndrome: A new technique for safe, endoscopic PEG removal.
Gastrointest Endosc 1995;41:508.
-
Saltzberg DM, Anand K, Juvan P, Joffe I. Colocutaneous fistula:An unusual complication of percutaneous endoscopic gastrostomy.
JPEN J Parenter Enteral Nutr. 1987;11:86–7.
-
Berger SA, Zarling EJ. Colocutaneous fistula following migrationof PEG tube.
Gastrointest Endosc. 1991;37:86–8.
-
Galat, SA, Gerig, KD, Porter, JA, Slezak FA. Management ofpremature removal of percutaneous gastrostomy.
Am Surg.1990;56:733–6.
-
Williams, E, Sabol, DA, Delegge, M. Small bowel obstruction causedby bowel wall hematoma after PEG.
Gastrointest Endosc.2003;57:273–4.
-
Chaer RA, Rekkas D, Trevino J, Brown R, Espat J.. Intrahepaticplacement of a PEG tube.
Gastrointest Endosc. 2003;57:763–5.
-
Chuang, CH, Chen, CY. Gastric herniation through PEG site.
Gastrointest Endosc. 2003;58:416.
-
Honjo S. A mother’s complaints of overeating by her 25-month-olddaughter: a proposal of anorexia nervosa by proxy.
Int J EatDisord. 1996;20:433–7.
-
Khurana V, Singh T. Percutaneous endoscopic gastrostomy sitemetastasis in esophageal cancer.
Gastrointest Endosc.2005;62:612.
-
Mincheff TV. Metastatic spread to a percutaneous gastrostomysite from head and neck cancer: case report and literature review.
JSLS. 2005;9:466–71.
-
Stiegmann, GV, Goff, JS, Silas, D, Pearlman N, Sun J, Norton L.Endoscopic versus operative gastrostomy: final results of aprospective randomized trial.
Gastrointest Endosc. 1990;36:1–5.
-
Vogt, W, Messmann, H, Lock, G, Gmeinwieser J, Feuerbach S,Schölmerich J, CT-guided PEG in patients with unsuccessfulendoscopic transillumination.
Dtsch Med Wochenschr.1996;121:359–63. German.
-
Bechtold ML, Matteson ML, Choudhary A, Puli SR, Jiang PP, RoyPK. Early Versus Delayed Feeding After Placement of aPercutaneous Endoscopic Gastrostomy: A Meta-Analysis.
Am JGastroenterol. 2008;103:2919–24.
-
Foutch PG, Talbert GA, Waring JP, Sanowski RA. Percutaneousendoscopic gastrostomy in patients with prior abdominal surgery:Virtues of the safe tract.
Am J Gastroenterol. 1988;83:147–50.
-
Bender JS. Percutaneous endoscopic gastrostomy placement inthe morbidly obese [letter].
Gastrointest Endosc. 1992;38:97–8.
-
Karhadkar AS, Naini P, Dutta SK. PEG-tube placement in a patientwith extreme obesity: overcoming the technical challenges.
Gastrointest Endosc. 2007;65:731–3.
-
Bochicchio GV, Guzzo JL, Scalea TM. Percutaneous endoscopicgastrostomy in the supermorbidly obese patient.
JSLS.2006;10:409–13.
-
Shaheen, NJ, Crosby, MA, Grimm, IS, Isaacs, K. The use ofpercutaneous endoscopic gastrostomy in pregnancy.
GastrointestEndosc. 1997;46:564–5.
-
Kynci, JA, Chodash, HB, Tsang, TK. PEG in a patient with ascitesand varices [letter].
Gastrointest Endosc. 1995;42:100–1.
-
Grant JP. Percutaneous endoscopic gastrostomy. Initial placementby single endoscopic technique and long-term follow-up.
Ann Surg.1993;217:168–74.
-
Rabeneck L, Wray NP, Petersen NJ. Long-term outcomes ofpatients receiving percutaneous endoscopic gastrostomy tubes.
J Gen Intern Med. 1996;11:287–93.
-
Amann W, Mischinger HJ, Berger A, Rosanelli G, Schweiger W,Werkgartner G, et al. Percutaneous endoscopic gastrostomy (PEG).8 years of clinical experience in 232 patients.
Surg Endosc.197;11:741–4.
-
Greif JM, Ragland JJ, Ochsner MG, Riding R. Fatal necrotisingfasciitis complicating percutaneous endoscopic gastrostomy.
Gastrointest Endosc. 1986;32:292–4.
-
Loser C, Wolters S, Folsch UR. Enteral long-term nutrition viapercutaneous endoscopic gastrostomy in 210 patients: a four-year prospective study.
Dig Dis Sci. 1998;43:2549–57.
-
So JB, Ackroyd FW. Experience of percutaneous endoscopicgastrostomy at Massachusetts General Hospital—indications andcomplications.
Singapore Med J. 1998;39:560–3.
-
Thakore JN, Mustafa M, Suryaprasad S, Agrawal S. Percutaneousendoscopic gastrostomy associated gastric metastasis.
J ClinGastroenterol. 2003;37:307–11.
-
Hosseini M, Lee JG. Metastatic esophageal cancer leading to gastricperforation after repeat PEG placement.
Am J Gastroenterol.1999;94:2556–8.
-
Dumortier J, Lapalus MG, Pereira A, Lagarrigue JP, Chavaillon A,Ponchon T. Unsedated transnasal PEG placement.
GastrointestEndosc. 2004;59:54–7.
-
Hull MA, Rawlings J, Morray FE. Audit of outcome of longtermenteral nutrition by percutaneous endoscopic gastrostomy.
Lancet.1993;341:869–72.
-
Ponsky JL, Gauderer MW, Stellato TA. Percutaneous endoscopicgastrostomy: a review of 150 cases.
Arch Surg. 1983;118:913–4.
-
Lin HS, Ibrahim HZ, Kheng JW, Fee WE, Terris DJ. Percutaneousendoscopic gastrostomy: strategies for prevention andmanagement of complications.
Laryngoscope. 2001;111:1847–52.
-
Biliary atresia (BA)It is a pathologic process leading to fibrosis and obliterationof the ductal system resulting in obstruction to bile flow, andcholestasis. The aetiology of this entity is unknown. Theincidence of BA is high in China and Japan (1:8,000) ascompared to Europe (1:17,000 live births), and is morecommon in girls than in boys.4-6 A higher incidence is found ininfants less than 32 weeks of gestational age, where thematernal age at conception is more than 35 years, and inmothers with parity 4 or more.7Venu RP, Brown RD, Pastika BJ, Erikson LW Jr. The buried bumpersyndrome: a simple management approach in two patients.
Gastrointest Endosc, 2002;56:582–4.
-
Walton GM. Complications of percutaneous endoscopicgastrostomy in patients with head and neck cancer—an analysisof 42 consecutive patients.
Ann R Coll Surg Engl.1999;81:272–6.
-
Segal D, Michaud L, Guimber D, Ganga-Zandzou PS, Turck D,Gottrand F. Late-onset complications of percutaneous endoscopicgastrostomy in children.
J Pediatr Gastroenterol Nutr.2001;33:495–500.
-
Rimon E. The safety and feasibility of percutaneous endoscopicgastrostomy placement by a single physician.
Endoscopy.2001;33:241–4.
-
Dwyer KM, Watts DD, Thurber JS, Benoit RS, Fakhry SM.Percutaneous endoscopic gastrostomy: the preferred method ofelective feeding tube placement in trauma patients.
J Trauma.2002;52:26–32.