|
Harshal S Mandavdhare, Amit Kumar, Vishal Sharma, Surinder S Rana Department of Gastroenterology, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
Corresponding Author:
Vishal Sharma Email: docvishalsharma@gmail.com
Abstract
Abdominal cocoon, variously described as encapsulating peritoneal sclerosis (EPS) or sclerosing encapsulating peritonitis or peritonitis chronica fibrosa incapsulata, represents a syndrome of clinical symptoms related to formation of a fibro-collagenous peritoneal membrane that involves the small intestinal loops. It is the outcome of several different etiologic processes. The major factors associated with development of an abdominal cocoon include continuous ambulatory peritoneal dialysis, tuberculosis, post-renal or liver transplant, use of beta-blockers although a proportion are of idiopathic origin. It is believed that the pathogenesis is driven by epithelial-to-mesenchymal transition of the mesothelial cells. The clinical presentation is related to development of altered gut motility resulting in abdominal pain and features of intestinal obstruction. Surgery is usually required in cases of unremitting intestinal obstruction. Although computed tomography may provide a pre-operative diagnosis, many cases are diagnosed at the time of surgery. Role of conservative therapy using drugs like steroids, tamoxifen, and mechanistic target of rapamycin (mTOR) inhibitors, or antitubercular therapy (in patients with tuberculosis) is uncertain. Early management aimed at the underlying etiological factor may ameliorate the symptoms and halt progression to formation of frank cocoon. Occasionally, conditions like peritoneal carcinomatosis or peritoneal encapsulation may mimic EPS.
|
48uep6bbphidcol2|ID 48uep6bbphidvals|1730 48uep6bbph|2000F98CTab_Articles|Fulltext Introduction
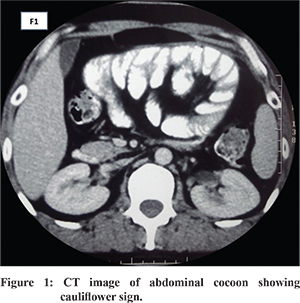
Abdominal cocoon or “Encapsulating peritoneal sclerosis” (EPS) was previously termed as sclerosing encapsulating peritonitis (SEP) and is an uncommon cause of intestinal obstruction. It was first described in 1907 by the term “peritonitis chronica fibrosa incapsulata” and later in 1978 after its detailed description as “abdominal cocoon”. 1 It is believed to be a result of a chronic intra-abdominal fibro-inflammatory process that results in formation of fibrous tissue sheets that cover, fix and ultimately constrict the gut compromising its motility. This eventually leads to a marbled, thickened, leathery fibro-connective tissue sheath like structure that envelopes the small intestine in the form of a cocoon ( Figure 1). Various terminologies have been used however EPS seems the most appropriate as it is self-descriptive with encapsulation of intestine resembling a cocoon and damage to the peritoneal membrane (peritoneal sclerosis). In advanced cases, inflammatory features are not always apparent, so the use of word peritonitis as in SEP is not preferred. 2 Indeed this name has been proposed as the appropriate term by the international society for peritoneal dialysis (ISPD) when cocoon formation or related changes occur in setting of the peritoneal dialysis. 3 However, it may be prudent to use this term in all clinical situations as the process seems to be the same result of different predisposing factors. Due to its nonspecific presentation during initial stages, before cocooning has fully developed, and non-availability of markers for early identification one needs to have a high index of suspicion in a given clinical scenario to reach a diagnosis. The present review will discuss the etiology, pathogenesis, pathology, clinical presentation, diagnostic evaluation, and treatment strategies with summary through management algorithm and concludes with the preventive strategies for this enigmatic condition.
Etiology
In its first description by Foo et al in 1978 in adolescent girls from tropical and sub-tropical areas it was called primary or idiopathic EPS as the etiology was obscure. 1 This was proposed to be retrograde peritonitis from spread of infection though fallopian tubes and retrograde menstruation with subsequent immunologic damage. However, later as it was also described abundantly in men, premenopausal females and children there seem little credence to this hypothesis. 4-6 A possibility of developmental abnormality cannot be ruled out, as abdominal cocoon has been associated with omental hypoplasia and mesenteric vascular malformation. 7 Apart from the primary variant, EPS is classified as secondary when there are identifiable underlying triggering factors that may include continuous ambulatory peritoneal dialysis (CAPD) with recurrent peritonitis which can be due to bacterial infection or sterile chemical peritonitis, peritoneal tuberculosis, use of certain drugs like beta blockers especially the historical drug practolol, methotrexate, asbestos, use of intraperitoneal chemotherapy, LeVeen shunt, ventriculoperitoneal shunts, systemic lupus erythematosus, sarcoidosis, luetinized the coma of ovary, ruptured dermoid cyst among others. 8 Besides these there have been reports of various rare and uncommon etiologies for cocoon summarized in Table 1. 9-18

A close differential for EPS is peritoneal encapsulation. It’s a congenital abnormality where in the small bowel is enclosed in a sac of accessory peritoneum derived from yolk sac due to abnormal return of the physiological umbilical hernia in the abdominal cavity during the twelfth week of gestation. It can involve part or whole of the small intestine. It’s usually an incidental finding during laparotomy for some other indication and rarely can present with intestinal obstruction, gangrene or aortic occlusion. If encountered in symptomatic patients it needs to be excised with lysis of inter-loop adhesions if present. 19,20
Pathogenesis
The peritoneal stromal and mesothelial reaction to the various etiologic factors for EPS results in inflammation giving way to peritoneal sclerosis or fibrosis that finally leads to cocoon formation. Chronic irritation of the peritoneum whether chemical or infective, disrupts the mesothelial cell junction damaging the subserosal tissue setting an inflammatory response ending in fibrosis. 21 In an experiment, household bleach was used in rats to start chemical peritonitis and later blood was instilled, the initial event caused membrane damage and the later lead to clot formation bringing the visceral surfaces together. This demonstrated that two events brought fibrosis, coagulation and inflammation together leading to cocoon formation. 22 Analogously recently the pathogenesis of EPS has been explained by the “Two Hit hypothesis” that states that two factors are required for onset of EPS. 2 Pathogenic mechanisms which contribute to genesis of EPS are shown in Figure 2. 21,23,24
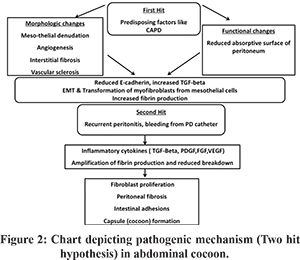
A predisposing factor that causes functional and morphological damage to the peritoneum like in peritoneal dialysis, chronic exposure to the dialysate results in mesothelial disruption (first hit) setting the ground for further development of sclerosis. An initiating factor in the form of an inflammatory stimuli superimposed on the damaged peritoneum like in recurrent peritonitis (second hit) in the setting of PD. The characteristics of the dialysate like acidity, high glucose and advanced glycation end products, hyper-osmolarity etc cause the first hit by causing mesothelial denudation and subsequent upregulation of profibrotic growth factors like TGF-beta, platelet derived growth factor (PDGF), tumour necrosis factor (TNF)-alpha setting the stage for fibrosis. 24 Also under the influence of transforming growth factor (TGF)-beta, mesothelial cells express plasminogen activator inhibitors (PAI) 1 and 2 leading to decrease in fibrin degradation. 24 Also, exposure to drugs like practolol has been shown to cause mesothelial cell disruption (first hit) that leads to the cascade of events leading to EPS. 23 The damage to mesothelium causes down-regulation of E-cadherin with loss of cell to cell contact and apical basal polarity and with the upregulation of TGF-beta there is epithelial to mesenchymal transformation with mesothelial cells getting converted to myofibroblasts with increased production of fibrin. 24 The second hit in the form of recurrent peritonitis cause upregulation of pro-inflammatory cytokines Interleukin 1, 6, 18, TNF-alpha which along with TGF-beta further expand the process of fibrosis. TGF-beta also causes over-expression of matrix metalloproteinase 2 and tissue inhibitor of matrix metalloproteinase 1 that inhibit fibrin degradation and along with vascular endothelial growth factor (VEGF), PDGF, fibroblast growth factor (FGF) lead to encapsulation and cocoon formation. 21,23,24
Pathology
The gross appearance is of a cocoon like encasement of the intestine. Three types of abdominal cocoon have been described based on the extent of involvement of the small intestine or other organs. If the membrane involves only a part of small intestine the cocoon is of type I, if the entire small bowel is involved it is the type II and if the colon or any visceral organs are also encapsulated then it is of type III.8 Recently, a type IV cocoon has been described with neuroendocrine tumors where in the entire peritoneum lining the abdominal cavity is involved by the cocoon. 15 However, this can also be regarded as an advanced type III cocoon. On histological examination the membrane shows lamellar matrix of fibrin suggesting that it is derived from the fibrin that has exuded during the “first hit”. The evidence for the “second hit” can be appreciated in form of blood and inflammatory cell infiltrate from recurrent peritonitis. 2
Clinical presentation
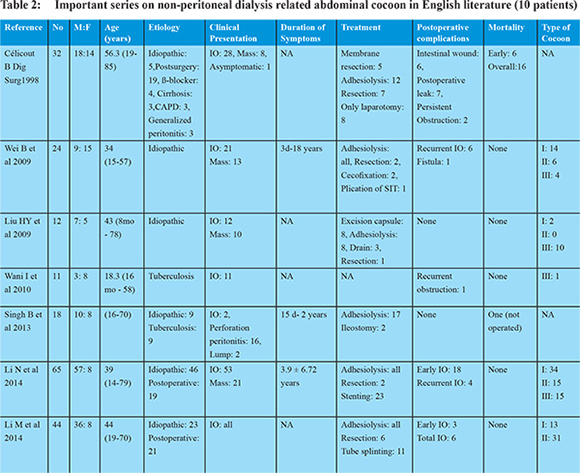
It is encountered equally in both genders and presents over a wide age range of ages from young as well as very old with oldest patient reported of 90 years. 5,6,25,26 The hallmark of EPS is the intermittent nature of its presentation. The initial symptoms are usually related to the altered gut motility and transit and altered peritoneal permeability. With the development of complete sclerosis due to formation of cocoon there are overt signs of intestinal obstruction (partial/ complete). A retrospective Japanese study proposed four stages of EPS in the setting of PD.27 The stage I is the pre-EPS stage where in due to increased peritoneal permeability patient develops ascites with hypoproteinemia. The Stage II presents with signs and symptoms of inflammation from bacterial or chemical peritonitis and the patient presents with fever, loss of appetite, loss of weight, fatigue, ascites with increase in acute inflammatory markers like ESR and CRP. With repeated attacks of stage II, the stage III sets in with progressive encapsulation with small intestinal obstruction and presents with abdominal pain, nausea, vomiting, constipation, and ascites with or without abdominal mass with signs of severe malnutrition. Finally after years, the stage IV sets with formation of cocoon and patient presents with complete ileus and abdominal mass. Since this description is primarily in the setting of PD, all stages are not likely to be seen in patients with other underlying etiological factors and is not uncommon for these patients to present directly in complete intestinal obstruction. Besides these features, patients may have telltale signs of the underlying etiology. Table 2 lists the important series that report about non-CAPD related cases of abdominal cocoon. 5,6,25,28-31
In patients undergoing peritoneal dialysis, encapsulating peritoneal sclerosis can be suspected in early stages when there is a hemorrhagic effluent from PD catheter or when elevated levels of inflammatory mediators and markers of the coagulation-fibrinolysis system such as interleukin-6 and fibrin/fibrinogen degradation products are raised. 32
Evaluation
The diagnosis of cocoon abdomen has to be suspected in appropriate clinical setting taking into account past medical and surgical history, along with findings on radiology. Although the idiopathic form of disease was initially recognized only in post-menarche female patients, now primary idiopathic variant is recognized to occur in any age and gender while several secondary causes of EPS are also recognized. 1,8 A study also reported a set of clinical features to identify cocoon preoperatively. These were: intestinal obstruction in a young girl without a definite etiology, presentation with abdominal pain and vomiting but no definite obstruction, history of previous spontaneously resolving episodes of obstruction and presentation with soft non tender abdominal lump. 33,34 These may not hold true for the patients with secondary EPS or even for all patients with primary EPS. Table 1 lists various etiologic factors that must be sought in a patient with abdominal cocoon.
Radiological evaluation
Various radiological modalities employed for diagnosis are abdominal X-rays, ultrasonography (USG), small bowel barium studies, contrast enhanced computed tomography (CECT) and contrast-enhanced magnetic resonance imaging (MRI). 6,35-37 These may help in establishing a convincing preoperative diagnosis in most (but not all) cases. On abdominal X-rays, dilated small bowel loops with multiple air fluid levels with clumping of bowel loops in center of abdomen may be seen, but the sensitivity is low5,38 Peritoneal and bowel wall calcification has been also reported. 39 Ultrasonography (USG) in abdominal cocoon may show dilated small bowel loops fixed to posterior abdominal wall, trilaminar appearance of bowel wall, ascites with or without loculations, a membrane covering the small bowel loops with occasional formation of a mass of bowel loops and adhesions. On USG clumped bowel loops appear to be in “concertina shape” attached with a narrow mesentery giving appearance like a “cauliflower”. 40,41 Ultrasound may be limited by gas in the bowel and is operator dependent. USG may also show “sandwich appearance” due to the presence of echogenic membrane around the bowel loops. 42 On small intestinal barium series, small intestine loops appear clumped towards center of abdomen because of membrane formation and appear like a cauliflower or accordion - first described by Navani in 1995. 40,43,44 There may also be prolonged transit time and in cases presenting with acute obstruction contrast studies may not be possible. 45 Computed tomography (CT) of abdomen has a sensitivity of 73-95% to detect etiology of high grade small bowel obstruction. 46 It is the most important modality short of surgery which helps in diagnosing the disease, delineating the extent of involvement and associated complications and helps in guiding management. The characteristic feature is conglomeration of small bowel loops in center encased by dense capsule and relatively contrast free periphery. CT may show ascites, loculated fluid collections, peritoneal and mesenteric thickening, features of intestinal obstruction, small bowel thickening, lymphadenopathy, mural or peritoneal calcifications. 5,34 Calcification occurs around the blood capillaries and may extend into the serosal and muscular layers. 47 The characteristic appearance of tethered small bowel loops because of retraction of the mesentery has been described as “ gingerbread man” sign. 48 Also, cauliflower sign is also described on CT ( Figure 1). The distinctive findings on CT described in EPS secondary to peritoneal dialysis are peritoneal thickening (100%), loculated fluid collection (90%), calcification (70%), congregated small bowel loops in the center of the abdomen (60%) and peritoneal enhancement (50%). 39 On CT it may not be always possible to see the fibrous capsule especially when it is thin. 49 CT scan in addition to accurately diagnosing the condition also rules out other differentials. In peritoneal encapsulation CT scan may reveal helical pattern of small bowel loops displaced anteriorly suggestive of “Helix sign” helpful in identifying peritoneal encapsulation as a differential of EPS. 50 Magnetic resonance imaging (MRI) has been recently reported as another useful modality in diagnosing EPS. The authors noted no significant difference between MRI and CT, moreover encasing membrane was more obvious in MRI with definite advantage of no exposure to ionizing radiation. 37 Recently use of “cine MRI” has been reported in diagnosis of cocoon in patients on chronic peritoneal dialysis where it was seen that that bowel movements in these patients, are confined to some areas of the abdomen. 51
Differential diagnosis
In any patient presenting with features suggestive of intestinal obstruction, possibility of (i) extra-luminal causes (extraintestinal or intramural) like: adhesions, masses (appendicitis, diverticulitis, peritoneal carcinomatosis, neuroendocrine tumor, lymphma), strangulation, hernia, malrotation and (ii) intraluminal causes like: intestinal tuberculosis, Crohn’s disease, intussusception, radiation enteropathy, bezoars need to be considered. The disease processes with similar imaging features like cocoon are internal hernia, pseudomyxoma peritonei, peritoneal carcinomatosis, peritoneal mesothelioma, sclerosing malignant lymphoma and malignant primary mesenteric tumors. 5,6,48 Patients, especially in areas with high prevalence of tuberculosis, should be evaluated with chest X-ray, sputum for AFB, skin PPD, erythrocyte sedimentation rate, ascitic fluid analysis including adenosine deaminase, serum ACE levels. Tubercular peritonitis may occur in three forms - wet, dry or fibrotic. CT in wet form will have ascites, which may be loculated. In fibrotic type matted bowel loops are seen with mesenteric nodules. In dry type there are fibrous adhesions and mesenteric thickening. Abdominal lymphadenopathy is another characteristic feature. Table 3 shows the various differential diagnosis of abdominal cocoon. 52-57
Management of abdominal cocoon
Prevention of EPS
The duration of CAPD is the most important risk factor which predisposes to occurrence of EPS in patients with chronic kidney disease on peritoneal dialysis. Also, repeated episodes of peritonitis, the type of peritoneal fluid used, faster peritoneal membrane transport, age at which PD was started, diminishing ultrafiltration and renal transplantation are recognized as other risk factors. 58 Since the consequences of EPS are devastating with high mortality rates, it is important to recognize and prevent this complication. Diminishing ultrafiltration may identify the subset of patients likely to develop EPS and may benefit from stopping PD. However, this needs to be confirmed on prospective studies and it may be worthy to recognize the role of bio-compatible fluids and concomitant use of anti-fibrotic agents in prevention of EPS. Preemptive use of tamoxifen in patients with PD related peritoneal sclerosis vis-à-vis no treatment reduced the occurrence of the advanced EPS and therefore seemed to reduce mortality related to EPS. This suggests that pre-emptive tamoxifen may be considered in patients with PD related peritoneal sclerosis who have not yet developed complete encapsulation. 59
Correction of underlying etiology
If the underlying cause is related to use of peritoneal dialysis, cessation of peritoneal dialysis and shifting to hemodialysis should be done. Removal of PD catheter should be considered, however occasionally withdrawal of PD may worsen the EPS. 58 Also, cessation of PD does not entirely eliminate the risk and EPS may occur even after renal transplantation. Similarly, the institution of anti-tubercular therapy may help in resolution/abatement of cocoon formation in tubercular abdominal cocoon (TAC). However, most available literature reports the use of surgical therapy to treat TAC and evidence regarding use of ATT alone or with immunosuppressive drugs like steroids is limited. In drug related EPS, discontinuation of drug may be beneficial.
Medical therapy
Several reports describe the use of various pharmacological agents for treatment of EPS. The agents used include steroids, mTOR inhibitors and tamoxifen. Corticosteroids have immunosuppressive and anti-inflammatory properties, which may be responsible for the benefit they exert in management of EPS. Multiple reports indicate the utility of steroids as a sole agent and in combination with other immunomodulators and with tamoxifen in management of EPS related to PD. Steroids are also beneficial for treatment of post-renal transplant EPS. 60 Tamoxifen is a selective estrogen receptor modulator (SERM) which has been reported to be of utility in therapy of EPS related to peritoneal dialysis. The benefit may be mediated by non-ER dependent mechanisms including modulation of TGF-beta related pathways. 58 Tamoxifen is also recognized to be of utility in management of other fibrosing disorders like sclerosing mediastinitis, retroperitoneal fibrosis.58 However, these patients also received surgical intervention in form of laparotomy with or without adhesiolysis. 61 In a large retrospective Dutch study, the subgroup of patients who received tamoxifen had a significantly lower mortality than those who did not receive the drug. 62 Some reports do suggest the benefit of tamoxifen monotherapy in doses of 10-40 mg/day. The fact that tamoxifen has no immunosuppressive action makes it convenient to use in most clinical situations. 63 In a recent review of published case series and reports, the use of inhibitors of mechanistic target of rapamycin (mTOR) like everolimus was reported in 20 patients with PD related encapsulating peritoneal sclerosis and suggested some benefit to patients who had developed EPS after renal transplant, possibly due to the fibrotic action of calcineurin inhibitors. 64 In another report, EPS was detected at a mean of 10.5 months after renal transplantation and of the 10 patients with post-transplant EPS, 5 were treated with mTOR inhibitors and four had a positive outcome. 65 Therefore, a change from calcineurin inhibitors (CNI) to mTORinhibitors may be considered in patients who develop EPS after renal transplantation.64 However, most reports have used the drug usually in combination with steroids and/or surgery. It is uncertain if use of medical treatment alone can suffice. The evidence regarding other immunomodulators is even less convincing. There is some experimental evidence from animal studies and occasional case reports that mycophenolate mofetil and azathioprine may have a role in treatment of EPS, however firm clinical evidence is lacking. 66 There is also evidence that CNI (cyclosporine and tacrolimus) may increase the risk of EPS. The increasing recognition of post renal transplantation EPS suggested that lowering of steroid dose and use of profibrotic CNI may have contributed to the trend. 67 The patients who develop post-transplant EPS may benefit by decreasing or eliminating the CNI. 64 A report also suggests that use of far-infrared therapy, through anti-inflammatory action and stimulation of neoangiogenesis and improved endothelial function through eNOS and HO-1, may improve EPS. 68 In spite of growing evidence of the utility of these agents in treatment of PD related EPS, the evidence in other forms of cocoon is virtually non-existent. Also, these pharmacological agents have often been used in varying combinations and in conjunction with surgical therapy. Therefore, no firm recommendations are possible regarding the dosage, duration and combination in which they may be used, especially in a non-PD EPS setting.
Surgical treatment
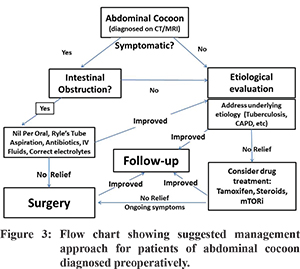
Traditionally in EPS, surgery has played both diagnostic and therapeutic role as has been seen in various series that most of the patients were diagnosed intra-operatively. 5,6,30 The most widely used surgical procedure is complete resection of membrane and wherever possible adhesiolysis should be performed cautiously avoiding inadvertent bowel injury. In case of injury during stripping and adhesiolysis the proximal most part of the intestine is exteriorized till recovery. Intestinal resection is only performed in non-viable gut but there are very high chances of post-operative fistula formation. To prevent post-operative recurrent obstruction, intestinal intubation or plication can be done retrograde through the appendicular orifice avoiding acute angulations, which also helps to minimize fistula formation. 5,31 A good preoperative nutritional support, enteral or parenteral, reduces post-operative complications, hospital stay and time to oral intake. 5 With the increasing awareness of the condition, pre-operative diagnosis can be made with help of CT scan with findings in the form of peritoneal thickening, calcification and enhancement, loculated ascites and bowel obstruction. However, these are findings seen in the advanced stage of the disease where the prognosis is poor with high mortality. 69 CT scan has a poor sensitivity in picking up the early membrane changes before EPS has formed and hence the role of laparoscopy in a patient presenting with recurrent abdominal pain with a background of known risk factors. Laparoscopy may show early changes of EPS in the form of peritoneal tanning and brown leathery appearance. With the use of tamoxifen, azathioprine and steroids the above finding have been shown to resolve with normalization of peritoneum. 69 A recent report from India suggests that the use of antitibercular therapy in patients with tubercular abdominal cocoon may help avoid surgery in a subset of patients. 70 Figure 3 depicts the suggested management strategy for patients in whom abdominal cocoon is recognized pre-operatively.
Future targets
The key to avoiding the formation of abdominal cocoon may lie in the prevention of epithelial-to-mesenchymal transition of the mesothelial cells of the peritoneum. This might avoid the genesis of peritoneal fibrosis consequent to peritoneal infection and/or inflammation. Various agents which have been tested in preclinical studies and may have anti-fibrotic properties include melatonin, rapamycin, pirfenidone. 70-72 Blockage of toll like receptors 2 and 4 have been proposed as potential therapeutic targets for PD associated peritoneal sclerosis as these are responsible for mediating the pro-fibrotic response in relation to infection and/or inflammation. 73 Other reported mediators for the fibrogenic response include angiotensin II receptors, vascular endothelial injury. 74 A study in mouse model has suggested the role of beta-blocker nebivolol in preventing peritoneal dialysis induced fibrogenic responses. 75 Other drugs and agents which have been found to have some benefit in animal studies include rosiglitazone, N-acetyl cysteine, colchicine, angiotensin inhibition, thalidomide as well as biocompatible PD solutions. 60 However, the translation of these experimental evidence into clinical practice is awaited. In conclusion, EPS is an enigmatic clinical entity which may result from a myriad of conditions and needs to be identified early to possibly delay the progression and avoid surgery. However in patients with unremitting symptoms and intestinal obstruction surgery remains the cornerstone to management.
References - Foo KT, Ng KC, Rauff A, et al. Unusual small intestinal obstruction in adolescent girls: the abdominal cocoon. Br J Surg. 1978;65:427-30.
- Honda K, Oda H. Pathology of encapsulating peritoneal sclerosis. Perit Dial Int. 2005;25 Suppl 4:S19-29.
- Kawaguchi Y, Kawanishi H, Mujais S, et al. Encapsulating peritoneal sclerosis: definition, etiology, diagnosis, and treatment. International Society for Peritoneal Dialysis Ad Hoc Committee on Ultrafiltration Management in Peritoneal Dialysis. Perit Dial Int. D2000;20 Suppl 4:S43-55.
- Akbulut S. Accurate definition and management of idiopathic sclerosing encapsulating peritonitis. World J Gastroenterol. 2015;21:675-87.
- Li N, Zhu W, Li Y, et al. Surgical treatment and perioperative management of idiopathic abdominal cocoon: single-center review of 65 cases. World J Surg. 2014;38:1860-7.
- Wei B, Wei HB, Guo WP, et al. Diagnosis and treatment of abdominal cocoon: a report of 24 cases. Am J Surg. 2009;198:348-53.
- Xu P, Chen LH, Li YM. Idiopathic sclerosing encapsulating peritonitis (or abdominal cocoon): a report of 5 cases. World J Gastroenterol. 2007;13:3649-51.
- Machado NO. Sclerosing Encapsulating Peritonitis: Review. Sultan Qaboos Univ Med J. 2016;16:e142-51.
- Anantha RV, Salvadori MI, Hussein MH, et al. Abdominal cocoon syndrome caused by Mycobacterium bovis from consumption of unpasteurised cow’s milk. Lancet Infect Dis. 2015;15:1498.
- Fei X, Yang HR, Yu PF, et al. Idiopathic abdominal cocoon syndrome with unilateral abdominal cryptorchidism and greater omentum hypoplasia in a young case of small bowel obstruction. World J Gastroenterol. 2016;22:4958-62.
- Yamada S, Tanimoto A, Matsuki Y, et al. Sclerosing encapsulating peritonitis (abdominal cocoon) associated with liver cirrhosis and diffuse large B-cell lymphoma: autopsy case. Pathol Int. 2009;59:681-6.
- Santos VM, Barbosa ER, Jr., Lima SH, et al.Abdominal cocoon associated with endometriosis. Singapore Med J. 2007;48:e240-2.
- M U, Kumar V, R RA, et al. Perforated GIST in Jejunum - A Rare Cause of Abdominal Cocoon. J Clin Diagn Res. 2014;8:132-3.
- K A, S V, C G, et al. Sclerosing peritonitis occurring in association with juvenile granulosa cell tumour - a cause of concern. J Clin Diagn Res. 2014;8:123-4.
- Wang YZ, King H, Diebold A. Cocoon formation in patients with midgut neuroendocrine tumors: a rare and unrecognized final pathway. Pancreas. 2013;42:944-8.
- Kaur S, Doley RP, Chabbhra M, et al. Post trauma abdominal cocoon. Int J Surg Case Rep. 2015;7c:64-5.
- Mohd Noor NH, Zaki NM, Kaur G, et al.Abdominal cocoon in association with adenomyosis and leiomyomata of the uterus and endometriotic cyst : unusual presentation. Malays J Med Sci. 2004;11:81-5.
- Tarquini R, Colagrande S, Rosselli M, et al. Complete resolution of primary sclerosing peritonitis (“abdominal cocoon”) following long term therapy for Tropheryma whipplei: a case report and review of literature. BMJ Case Rep. 2009;2009.
- Naidoo K, Mewa Kinoo S, Singh B. Small Bowel Injury in Peritoneal Encapsulation following Penetrating Abdominal Trauma. Case Rep Surg. 2013;2013:379464.
- Lifschitz O, Tiu J, Sumeruk RA. Peritoneal encapsulation of small intestine. A case report. S Afr Med J. 1987;71:452.
- Hoff CM. Experimental animal models of encapsulating peritoneal sclerosis. Perit Dial Int. 2005;25 Suppl 4:S57-66.
- Levine S, Saltzman A. Abdominal cocoon: an animal model for a complication of peritoneal dialysis. Perit Dial Int. 1996;16:613-6.
- Dobbie JW. Pathogenesis of peritoneal fibrosing syndromes (sclerosing peritonitis) in peritoneal dialysis. Perit Dial Int. 1992;12:14-27.
- Moinuddin Z, Summers A, Van Dellen D, et al. Encapsulating peritoneal sclerosis-a rare but devastating peritoneal disease. Front Physiol. 2014;5:470.
- Celicout B, Levard H, Hay J, et al. Sclerosing encapsulating peritonitis: early and late results of surgical management in 32 cases. French Associations for Surgical Research. Dig Surg. 1998;15:697-702.
- Yavuz R, Akbulut S, Babur M, et al. Intestinal Obstruction Due to Idiopathic Sclerosing Encapsulating Peritonitis: A Case Report. Iran Red Crescent Med J. 2015;17:e21934.
- Nakamoto H, Kawaguchi Y, Suzuki H. Encapsulating peritoneal sclerosis in patients undergoing continuous ambulatory peritoneal dialysis in Japan. Adv Perit Dial. 2002;18:119-23.
- Liu HY, Wang YS, Yang WG, et al. Diagnosis and surgical management of abdominal cocoon: results from 12 cases. Acta Gastroenterol Belg. 2009;72:447-9.
- Wani I, Ommid M, Waheed A, et al. Tuberculous abdominal cocoon: original article. Ulus Travma Acil Cerrahi Derg. 2010;16:508-10.
- Singh B, Gupta S. Abdominal cocoon: a case series. Int J Surg. 2013;11:325-8.
- Li M, Zhu W, Li Y, et al. Long intestinal tube splinting prevents postoperative adhesive small-bowel obstruction in sclerosing encapsulating peritonitis. BMC Gastroenterol. 2014;14:180.
- Kawanishi H, Watanabe H, Moriishi M, et al. Successful surgical management of encapsulating peritoneal sclerosis. Perit Dial Int. 2005;25 Suppl 4:S39-47.
- Yip FW, Lee SH. The abdominal cocoon. Aust N Z J Surg. 1992;62:638-42.
- Awe JA. Abdominal cocoon syndrome (idiopathic sclerosing encapsulating peritonitis): how easy is its diagnosis preoperatively? A case report. Case Rep Surg. 2013;2013:604061.
- Maguire D, Srinivasan P, O’Grady J, et al. Sclerosing encapsulating peritonitis after orthotopic liver transplantation. Am J Surg. 2001;182:151-4.
- Wang Q, Wang D. Abdominal cocoon: multi-detector row CT with multiplanar reformation and review of literatures. Abdom Imaging. 2010;35:92-4.
- Jovani M, Baticci F, Bonifacio C, et al. Abdominal cocoon or idiopathic encapsulating peritoneal sclerosis: magnetic resonance imaging. Dig Liver Dis. 2014;46:192-3.
- Kaur R, Chauhan D, Dalal U, et al. Abdominal cocoon with small bowel obstruction: two case reports. Abdom Imaging. 2012;37:275-8.
- Candido Pde C, Werner Ade F, Pereira IM, et al. Sclerosing encapsulating peritonitis: a case report. Radiol Bras. 2015;48:56-8.
- Hur J, Kim KW, Park MS, et al.Abdominal cocoon: preoperative diagnostic clues from radiologic imaging with pathologic correlation. AJR Am J Roentgenol. 2004;182:639
- Ti JP, Al-Aradi A, Conlon PJ, et al. Imaging features of encapsulating peritoneal sclerosis in continuous ambulatory peritoneal dialysis patients. AJR Am J Roentgenol. 2010;195:W50-4.
- Garosi G. Different aspects of peritoneal damage: fibrosis and sclerosis. Contrib Nephrol. 2009;163:45-53.
- Serafimidis C, Katsarolis I, Vernadakis S, et al. Idiopathic sclerosing encapsulating peritonitis (or abdominal cocoon). BMC Surg. 2006;6:3.
- Navani S, Shah P, Pandya S, et al.Abdominal cocoon--the cauliflower sign on barium small bowel series. Indian J Gastroenterol. 1995;14:19.
- Ndiaye AR, Mbengue A, Soko TO, et al. Idiopathic sclerosing encapsulating peritonitis: a case in an adolescent girl. Diagn Interv Imaging. 2012;93:629-31.
- Burkill GJ, Bell JR, Healy JC. The utility of computed tomography in acute small bowel obstruction. Clin Radiol. 2001;56:350-9.
- Naniwadekar RG, Kulkarni SR, Bane P, et al. Abdominal cocoon: an unusual presentation of small bowel obstruction. J Clin Diagn Res. 2014;8:173-4.
- Tannoury JN, Abboud BN. Idiopathic sclerosing encapsulating peritonitis: abdominal cocoon. World J Gastroenterol. 2012;18:1999-2004.
- Jeong YJ, Kim S, Kwak SW, et al. Neoplastic and nonneoplastic conditions of serosal membrane origin: CT findings. Radiographics. 2008;28:801-17
- Mitrousias V, Alexiou E, Katsanas A, et al. The helix sign in the peritoneal encapsulation syndrome: a new sign in a rare cause of bowel obstruction? J Gastrointestinal Liver Dis. 2015;24:144.
- Wright B, Summers A, Fenner J, et al. Initial observations using a novel “cine” magnetic resonance imaging technique to detect changes in abdominal motion caused by encapsulating peritoneal sclerosis. Perit Dial Int. 2011;31:287-90.
- Bridda A, Padoan I, Mencarelli R, et al. Peritoneal mesothelioma: a review. MedGenMed. 2007;9:32.
- Akhan O, Kalyoncu F, Ozmen MN, et al. Peritoneal mesothelioma: sonographic findings in nine cases. Abdom Imaging. 1993;18:280-2.
- Levy AD, Shaw JC, Sobin LH. Secondary tumors and tumorlike lesions of the peritoneal cavity: imaging features with pathologic correlation. Radiographics. 2009;29:347-73.
- Hanbidge AE, Lynch D, Wilson SR. US of the peritoneum. Radiographics. 2003;23:663-84
- Sahoo SP, Gangopadhyay AN, Gupta DK, et al. Abdominal cocoon in children: a report of four cases. J Pediatr Surg. 1996;31:987-8.
- Lewin K, McCarthy LJ. Peritoneal encapsulation of the small intestine. Gastroenterology. 1970;59:270-2.
- Habib SM, Betjes MG, Fieren MW, et al. Management of encapsulating peritoneal sclerosis: a guideline on optimal and uniform treatment. Neth J Med. 2011;69:500-7.
- del Peso G, Bajo MA, Gil F, et al. Clinical experience with tamoxifen in peritoneal fibrosing syndromes. Adv Peritl Dial. 2003;19:32-5.
- Cornelis T, Oreopoulos DG. Update on potential medical treatments for encapsulating peritoneal sclerosis; human and experimental data. Int Urol Nephrol. 2011;43:147-56.
- Eltoum MA, Wright S, Atchley J, et al. Four consecutive cases of peritoneal dialysis-related encapsulating peritoneal sclerosis treated successfully with tamoxifen. Perit Dial Int. 2006;26:203-6.
- Korte MR, Fieren MW, Sampimon DE, et al. Tamoxifen is associated with lower mortality of encapsulating peritoneal sclerosis: results of the Dutch Multicentre EPS Study. Nephrol Dial Transplant. 2011;26:691-7.
- Guest S. Tamoxifen therapy for encapsulating peritoneal sclerosis: mechanism of action and update on clinical experiences. Perit Dial Int. 2009;29:252-5.
- Ghadimi M, Dashti-Khavidaki S, Khalili H. mTOR inhibitors for management of encapsulating peritoneal sclerosis: a review of literatures. Ren Fail. 2016:1-7.
- Messina M, Ariaudo C, Mella A, et al. mTOR inhibitors for medical treatment of post-transplantation encapsulating peritoneal sclerosis: a favourable single center experience. J Nephrol. 2015;28:245-9.
- Lafrance JP, Letourneau I, Ouimet D, et al. Successful treatment of encapsulating peritoneal sclerosis with immunosuppressive therapy. Am J Kidney Dis. 2008;51:e7-10.
- Fieren MW, Betjes MG, Korte MR, et al. Posttransplant encapsulating peritoneal sclerosis: a worrying new trend? Perit Dial Int. 2007;27:619-24.
- Ou SM, Hu FH, Yang WC, et al.Far-infrared therapy as a novel treatment for encapsulating peritoneal sclerosis. Am J Gastroenterol. 2014;109:1957-9.
- Kropp J, Sinsakul M, Butsch J, et al. Laparoscopy in the early diagnosis and management of sclerosing encapsulating peritonitis. Semin Dial. 2009;22:304-7.
- Sharma V, Mandavdhare HS, Rana SS, Singh H, Kumar A, Gupta R. Role of conservative management in tubercular abdominal cocoon: a case series. Infection. 2017 doi: 10.1007/s15010-017-1012-5.
- Xiang S, Li M, Xie X, et al. Rapamycin inhibits epithelial-to-mesenchymal transition of peritoneal mesothelium cells through regulation of Rho GTPases. FEBS J. 2016;283:2309-25.
- Bayhan Z, Zeren S, Kocak FE, et al. Antiadhesive and anti-inflammatory effects of pirfenidone in postoperative intra-abdominal adhesion in an experimental rat model. J Surg Res. 2016;201:348-55.
- Raby AC, Colmont CS, Kift-Morgan A, et al. Toll-Like Receptors 2 and 4 Are Potential Therapeutic Targets in Peritoneal Dialysis-Associated Fibrosis. J Am Soc Nephrol. 2016 Jul 18. pii: ASN.2015080923
- Morinelli TA, Luttrell LM, Strungs EG, et al. Angiotensin II receptors and peritoneal dialysis-induced peritoneal fibrosis. Int J Biochem Cell Biol. 2016;77:240-50.
- Liappas G, Gonzalez-Mateo G, Aguirre AR, et al. Nebivolol, a beta1-adrenergic blocker, protects from peritoneal membrane damage induced during peritoneal dialysis. Oncotarget. 2016;7:30133-46.
|