48uep6bbphidvals|251
48uep6bbphidcol4|ID
48uep6bbph|2000F98CTab_Articles|Fulltext
Xanthogranulomatous involvement of the gastrointestinal tract is rare, gall bladder is the commonest site affected. Rare involvement of the sigmoid colon, stomach and periampullary region have been reported. Most of these lesions impose preoperative diagnostic difficulties, malignancy being the most common misdiagnosis. Isolated cases of xanthogranulomatous gastritis is very rare and infiltration from an associated xanthogranulomatous cholecystitis is a possibility. We describe a diabetic patient with isolated xanthogranulomatous involvement of the stomach with chronic perforation and communication with the liver parenchyma.
Case report
A 51-year-old diabetic male patient presented with upper abdominal pain of moderate intensity for six months. He had had one discrete episode of severe, dull epigastric pain associated with low grade fever but no vomiting, requiring admission in another medical centre. There was no history of melena, jaundice or weight loss and the rest of his medical history was unremarkable. On examination he was found to have pallor, epigastric tenderness and mild hepatomegaly. Laboratory investigations showed a low hemoglobin level (9.8 gm%). Liver function tests and C-reactive protein level were normal. Screening for HIV, Hepatitis B and Hepatitis C were negative.
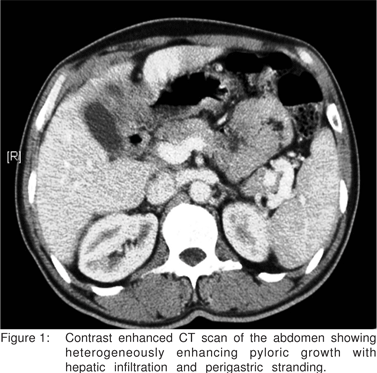
Abdominal ultrasonography revealed a heterogenous mass in the left lobe of the liver contiguous with a thickened pylorus. There was another well defined lesion in segment VII of the liver with cystic and necrotic areas. A contrast enhanced CT scan of the abdomen showed a heterogeneously enhancing pyloric growth with perigastric stranding (Figure 1). The lesion was infiltrating the liver and there was a cystic focal hepatic lesion, suspicious of metastasis. The gall bladder was normal. The rest of the abdominal and pelvic organs were normal.
Image guided fine needle aspiration cytology (FNAC) from the pyloro-hepatic mass was performed with three passes. The aspirate showed atypical cells, but no definite evidence of malignancy.
Esophago-gastroduodenoscopy showed a nodular friable mucosa in the anterior and superior wall of the antro-pyloric region of the stomach. The first and second parts of the duodenum were normal. Biopsy from antropyloric region showed no specific lesion.
At laparotomy, a pus-containing fistulous tract was seen arising from the anterior wall of the stomach, extending onto the inferior margin of segment IV of liver. A small perforation with thickening was noted in the region of the stomach adherent to the liver substance. No definite mass was palpable and there was no significant lymphadenopathy. The gall bladder was normal. The findings were suggestive of an inflammatory etiology rather than malignancy. A frozen section from this region was reported to show xanthomatous and granulomatous inflammation, with no evidence of malignancy. A distal gastrectomy, rather than a subtotal gastrectomy, as planned earlier, was performed. The cystic liver lesion in segment VII was not explored. He had an uneventful postoperative recovery.
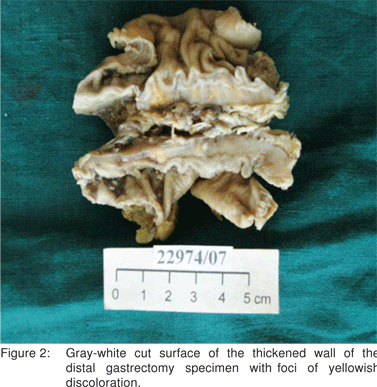
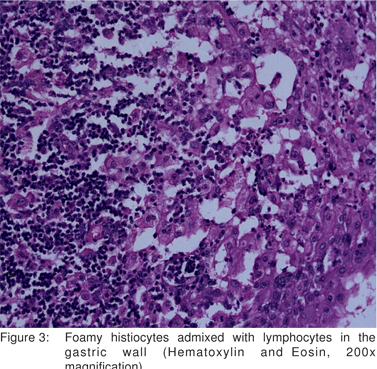
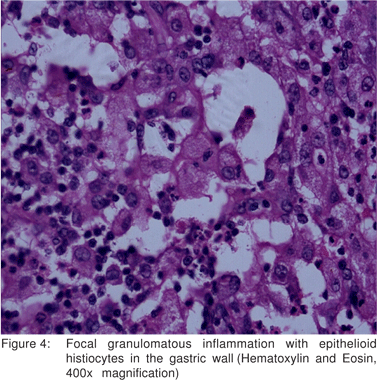
Pathological examination of the distal gastrectomy specimen showed diffuse thickening of the gastric wall. The cut surface was soft and gray white with yellowish foci extending into the attached adipose tissue. The adjacent mucosa appeared unremarkable (Figure 2). Microscopic examination revealed transmural infiltrates of lymphocytes, plasma cells, many lymphoid follicles, and numerous aggregates of foamy histiocytes (Figure 3). A few granulomas composed of epithelioid histiocyte and Langhans’ type multinucleate giant cells were also seen (Figure 4) but caseation, acid fast bacilli (AFB) or fungal organisms were not present. Gram stain did not show significant bacteria within the infiltrate. There was submucosal fibrosis and hypertrophy and fibrosis of the muscularis propria with neuronal hyperplasia. There was no evidence of malignancy. On immunohistochemistry, the foamy histiocytes expressed CD68 and were negative for cytokeratin and smooth muscle actin . Based on these findings a diagnosis of xanthogranulomatous gastritis was made.
The patient was well at 20 months’ follow up. Gastroscopy showed a jejunal stomal ulcer and biopsy from this site showed chronic active jejunitis with fibrosis. Separate biopsies from the gastrojejunostomy stomal site showed non-specific changes and no granulomas. AFB stain was negative.
Discussion
Xanthogranulomatous gastritis (XGG) is a rare disorderwith few case reports in literature[1,2,3]. In the gastrointestinal tract, the gall bladder is the most common site affected by xanthogranulomatous (XG) inflammation and a prevalence of 9% has been reported in a series of cholecystectomies from a large center in India[4,5]. Infiltration of adjacent organs can occur in xanthogranulomatous cholecystitis (XGC), mimicking gall bladder cancer[6]. Gastric involvement by XG inflammation has been reported to be secondary to extension from the gall bladder rather than a primary XGG[2,3], whose existence has even been questioned in literature[3].
Isolated XG inflammation has been reported to present as rapidly enlarging submucosal nodules in the stomach and in the sigmoid colon[1,7]. There is a report of XG inflammation causing an obstructive lesion in the sigmoid[8]. Other organs that can be affected by xanthogranulomatous inflammation include the kidney, bone, ovary, soft tissue, pancreas, lymph nodes and the periampullary region[3,4]. It is difficult to make an accurate preoperative diagnosis of xanthogranulomatous inflammation, an infiltrative malignancy is a common preoperative diagnosis[1,4,6,7]. Acute abdominal pain and fever in the index patient could be due to small, sealed perforation of the stomach, resulted in invasion of liver. Perforation of the gall bladder was observed at operation in XGC[9].
Other radiological differential diagnoses that have been considered with involvement of the sigmoid colon include pelvic inflammatory disease and actinomycosis.[7] Most patients require operative intervention because of symptoms, complications and an inconclusive preoperative diagnosis. Intraoperative frozen section examination was valuable however, to avoid an unnecessary radical resection, as has been emphasized in literature[7].
The precise pathogenesis of isolated XGG is unknown. When XGC and XGG co-exist, it is conceivable that deepening of the XG process in the gall bladder and adhesions may result in transmural involvement of the gastric wall[2]. A bile- reflux induced pathogenesis, like in XGC, has been implicated in XGG, especially when cholesterol crystals have been detected in the inflammatory infiltrate[2]. Bile reflux has also been presumed to have a role in the pathogenesis of gastric xanthoma, a more localized but similar lesion[10]. We did not find any evidence of bile reflux in the stomach. Sinus and fistula formation and the involvement of distant sites, although uncommon, are recognized complications of XG inflammation of the gall bladder, kidney and appendix[11,12]. Although sealed perforation of the stomach can present as a pseudotumor mimicking gastric malignancy, there is only one case report of gastric perforation associated with XG pseudotumor[13]. Rare cases of xanthogranulomatous cholecystitis with liver abscess[14] and XG pyelonephritis with secondary involvement of the liver have been reported[15].
References
1. Kubosawa H, Yano K, Oda K, Shiobara M, Ando K, Nunomura M, et al. Xanthogranulomatous gastritis with peudosarcomatous changes. Pathol Int. 2007;57:291–5.
2. Guarino M, Reale D, Micoli G, Tricomi P, Cristofori E. Xanthogranulomatous gastritis: association with xanthogranulomatous cholecystitis. J Clin Pathol . 1993;46:88–90.
3. Parsons MA. Xanthogranulomatous gastritis: an entity or a secondary phenomenon? J Clin Pathol. 1993;46:580–1.
4. Pottakkat B, Saxena R, Nag HH, Kumari N, Krishnani N. Ampullary xanthogranulomatous inflammation mimicking periampullary cancer: Report of a case. JOP. 2006;7:222–5.
5. Guzman-Valdivia G. Xanthogranulomatous cholecystitis: 15 years’ experience. World J Surgery. 2004;28:254–7.
6. Enomoto T, Todoroki T, Koike N, Kawamoto T, Matsumoto H. Xanthogranulomatous cholecystitis mimicking stage IV gallbladder cancer. Hepatogastroenterology. 2003;50:1255–8.
7. Oh YH, Seong SS, Jang KS, Chung YW, Paik CH, Park YW, et al. Xanthogranulomatous inflammation presenting as a submucosal mass of the sigmoid colon. Pathol Int. 2005;55:440–4.
8. Lo CY, Lorentz TG, Poon CS. Xanthogranulomatous inflammation of the sigmoid colon: a case report. Aust N Z J Surg. 1996;66:643–4.
9. Ladefoged C, Lorentzen M. Xanthogranulomatous cholecystitis. A clinicopathological study of 20 cases and review of the literature. APMIS. 1993;101:869–75.
10. Domellof L, Eriksson S, Helander HF, Janunger KG. Lipid islands in the gastric mucosa after resection for benign ulcer disease. Gastroenterology. 1977;72:14–8.
11. Roberts KM, Parsons MA. Xanthogranulomatous cholecystitis: clinicopathological study of 13 cases. J Clin Pathol. 1987;40:412–7.
12. Parsons MA, Harris SC, Longstaff AJ, Grainger RG. Xanthogranulomatous pyelonephritis: a pathological, clinical, and aetiological analysis of 87 cases. Diagn Histopathol. 1983;6:203–19.
13. Lai HY, Chen JH, Chen CK, Chen YF, Ho YJ, Yang MD, et al. Xanthogranulomatous pseudotumor of the stomach induced by perforated peptic peptic ulcer mimicking a stromal tumor. Eur Radiol. 2006;16:2371–2.
14. Eriguchi N, Aoyagi S, Horiuchi H, Tamae T, Uchida S, Hiraki M, et al. Xanthogranulomatous cholecystitis with liver abscess and metastatic endophthalmitis: report of a case. Surg Today. 2002;32:285–8.
15. Taskinen S, Giordano S, Rintala R. Xanthogranulomatous pyelonephritis infiltrating liver. J Pediatr Surg. 2008;43:e7–9.