Discussion
PHL is a rare entity, making up to 0.4% of extranodal non- Hodgkins lymphoma, and 0.01% of all non-Hodgkins lymphoma.[1] Since 1965, only about 100 cases have been reported worldwide.[1] Secondary involvement of the liver occurs more commonly in approximately 50-60% of patients with Hodgkin’s or non-Hodgkin’s lymphoma.[2] The incidence of hepatic involvement at presentation is much lower, however occurs largely in the due course of the disease. There is increased prevalence of lymphoma among individuals with HIV infection/AIDS and the clinical presentations and CT findings are often atypical compared with lymphomas in other patients.[3] In a large biopsy proven series, the most common hepatic neoplasm in patients with AIDS was lymphoma.[4]
On imaging (USG, CT, MRI) three well recognized morphologic patterns of lymphoma have been described.These are the diffuse infiltrative variety, the nodular variety, and a mixed infiltrative and nodular variety.[5] Infiltrative involvement is most common in the secondary lymphoma, and the imaging findings may range from a normal to an enlarged liver.[2] Hepatomegaly is however not a sensitive or a specific finding. Discrete nodular lesions are exceedingly uncommon in Hodgkin’s lymphoma, but occur in half of the patients with non-Hodgkin’s lymphoma. Nodular involvement may be detectable on imaging as single or multiple focal liver lesions.
Solitary liver mass lesion is the most common presentation of PHL, followed by multiple liver lesions.[2, 6] The lesions are of low echogenicity on USG, low density on CT, and low signal intensity on T1-weighted and high signal intensity on T2- weighted MR imaging.[2, 7] Most PHLs do not show any enhancement but patchy enhancement or an enhancing ring has been described in few cases.[6, 7] Mixed infiltrative and nodular involvement has a range of imaging features of the other two varieties, such as the presence of hepatomegaly and focal liver lesions.
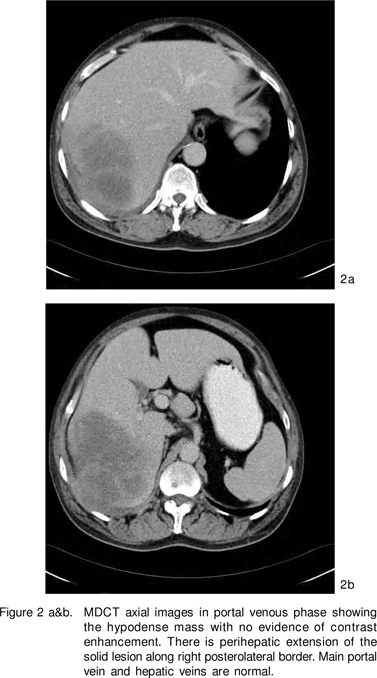
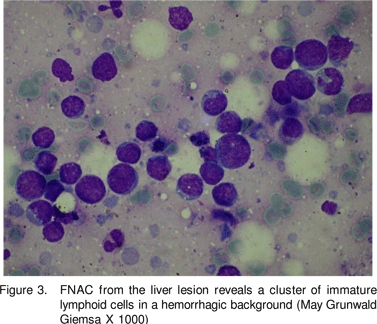
In our patient, there was no neovascularity of the lesion seen on arterial phase and no enhancement was seen on either arterial or portal venous phases of CT. The portal vein and its branches and hepatic veins were normal. As a result the hypervascular lesions like hemangioma, focal nodular hyperplasia, adenoma, hepatocellular carcinoma were excluded based on the enhancement pattern. The only differential diagnosis in a HIV positive patient based on the attenuation of the lesion and its hypovascular nature was an infectious pathology or a lymphoma. However infectious cause was less likely in the absence of thick shaggy, irregular walls of the lesion. Also there was perihepatic extension of the solid lesion without presence of any perihepatic fluid. Therefore a prospective diagnosis of a lymphoma could be made, which was confirmed on cytology.
In conclusion, biphasic MDCT helps in the diagnosis of PHL in a cases of HIV infection by showing the hypovascularity of the hepatic lesion and excluding any other organomegaly, focal lesions or lymphadenopathy.
References
1. Avlonitis VS, Linos D. Primary hepatic lymphoma. Eur J Surg.1999;165:725–9.
2. Gazelle GS, Lee MJ, Hahn PF, Goldberg MA, Rafaat N, Mueller PR. US, CT, and MRI of primary and secondary liver lymphoma. J Comput Assist Tomogr. 1994;18:412–5.
3. Nyberg DA, Jeffrey RB Jr, Federle MP, Bottles K, Abrams DI. AIDSrelated lymphomas: evaluation by abdominal CT. Radiology. 1986;159:59–63.
4. Poles MA, Dieterich DT, Schwarz ED, , Lew EA, Lew R, et al. Liver biopsy f indings in 501 pat ients infected with human immunodeficiency virus (HIV). J Acquir Immune Defic Syndr Hum Retrovirol. 1996;11:170–7.
5. Maher MM, McDermott SR, Fenlon HM, Conroy D, O’Keane JC, Carney DN, et al. Imaging of primary non-Hodgkin’s lymphoma of the liver. Clin Radiol. 2001;56:295–301.
6. Sanders LM, Botet JF, Straus DJ, Ryan J, Filippa DA, Newhouse JH. CT of primary lymphoma of the liver. AJR Am J Roentgenol. 1989; 152:973–6.
7. Memeo L, Pecorello I, Ciardi A, Aiello E, De Quarto A, Di Tondo U. Primary non-Hodgkin’s lymphoma of the liver. Acta Oncol. 1999; 38:655–8.