|
Kumble S Madhusudhan1, Premananda Pattanaik2, Nihar Ranjan Dash2, Sujoy Pal2, Vineet Ahuja3, Deep N Srivastava1, Peush Sahni2 Departments of 1Radiodiagnosis, 2Gastrointestinal Surgery and 3Gastroenterology, All India Institute of Medical Sciences, New Delhi, India.
Corresponding Author:
Dr Peush Sahni Email: peush_sahni@hotmail.com
Abstract
Aim: To compare multi-detector CT esophagography (MDCTE) in patients of esophageal carcinoma with barium swallow and upper gastrointestinal endoscopy. Materials and Methods: Seventy patients (44 males; mean age 55.1 years) of esophageal carcinoma were included in the study after informed consent. After an upper gastrointestinal endoscopy and biopsy, MDCTE and barium swallow study were performed on the same day. MDCTE was performed by manual instillation of room air via a nasogastric tube (NGT) inserted into the hypopharynx, and patient comfort assessed on a three-point scale. The MDCTE images were evaluated for the degree of distension and diagnostic quality. Kappa weighted analysis assessed concordance between MDCTE and conventional imaging (barium swallow and endoscopy). Results: In 68 (97%) patients, MDCTE was considered tolerable. Good or fair esophageal distension and diagnostic quality MDCTE study were achieved in 63 (90%) patients. The agreement between conventional studies (barium swallow and endoscopy) and MDCTE for the presence of lesion and lesion morphology was 91.3% and 63%, respectively. Conclusion: MDCTE provides diagnostic quality images with adequate distension of esophagus and better lesion definition in most patients without significant discomfort.
|
48uep6bbphidcol2|ID 48uep6bbphidvals|2952 48uep6bbph|2000F98CTab_Articles|Fulltext Introduction
Carcinoma of the esophagus is one of the common cancers of the alimentary tract, with an annual incidence of 7.5 to 10.3 per 100,000 population in India.1 The disease is aggressive with a dismal 5-year survival of 18%.2 With the increasing use of chemo-radiotherapy (CRT), the survival rates have increased to 42% with a reduced local recurrence rate.2,3 Accurate staging of the disease is important for prognostication and planning appropriate treatment.4 Neoadjuvant CRT changes the extent of the disease at surgery. Thus, accurate definition of the extent of the disease prior to treatment is of utmost importance.5 Currently, barium esophagogram and multi-detector computed tomography (MDCT) scans are used to stage esophageal carcinoma. The limitations of these techniques include an inadequate definition of tumor margins, inaccurate assessment of the T-and N-stage of the disease, and poor evaluation of response to neoadjuvant CRT.6,7 An accurate definition of the longitudinal extent of the tumor is essential for various reasons.8,9 These include the classification of involved lymph nodes as regional or metastatic, and planning the field for radiotherapy, the surgical approach, or the length and position of palliative stents. A recent study by Sillah et al10 suggested that esophageal tumor length defined by routine MDCT did not correlate with the pathological extent of the tumor. A collapsed esophagus overestimates tumor length (Figure 1). In routine MDCT chest, the esophagus is distended by the ingestion of an iodinated contrast agent before scanning. The distension is inconsistent due to the rapid transit of the contrast and depends on the density of contrast swallowed.11 MDCT esophagography (MDCTE)produces proper distension of the esophagus with air for better estimation of wall thickness, disease extent, and distensibility.12 Only a few studies are available in the literature evaluating esophageal carcinoma with MDCT esophagography (MDCTE), and only one study compared MDCTE with barium esophagogram.4,11,13-18 With this background, we aimed to assess the feasibility, comfort, and diagnostic accuracy of MDCTE in esophageal carcinoma and to compare the findings of MDCTE with barium swallow and upper gastrointestinal endoscopy.
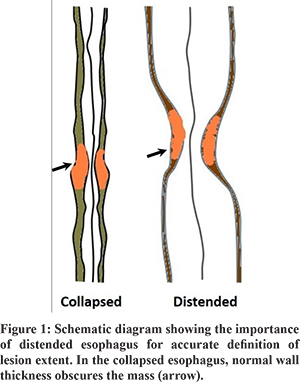
Materials and Methods
Patients
The Institute Ethics Committee approved the study. All consecutive endoscopically diagnosed cases of carcinoma of esophagus who presented to the gastroenterology, or gastrointestinal surgery departments were included after obtaining written informed consent. The inclusion criteria were treatment-naive biopsy diagnosed esophageal carcinoma. Patients with renal failure (in whom intravenous iodinated contrast could not be given), those with an allergy to iodinated contrast agents, and un-cooperative patients were excluded.
Methods
The patient was evaluated for symptoms of dysphagia, vomiting, hematemesis, weight loss, anorexia, and duration of symptoms. The upper gastrointestinal endoscopy (UGIE) images of the mass and the biopsy results of all patients were recorded. Subsequently, the patient underwent an MDCTE and barium esophagogram within a week of the UGIE.
MDCT Esophagography
MDCTE was done on a 40-slice CT scanner (Somatom Sensation, Siemens, Erlangen, Germany) after an overnight fast. A nasogastric tube (NGT, 8F) was inserted via the nostrils for 15-18 cm, such that its tip was below the cricopharyngeal sphincter. Once the patient was on the CT scan table, the proximal end of the NGT was connected through a long connector tubing and a three-way stop cock to a 50 ml syringe. The patient was instructed not to belch during the scan. Just before the scan, 10 mg of hyoscine butyl bromide was injected intravenously to relax the esophagus and reduce its peristalsis. Room air was instilled through the NGT 10 seconds before the scan (at a rate of 25-30 ml/sec) and continued until completion. MDCT scan was performed in the venous phase, 70 seconds after intravenous injection of the iodinated contrast agent. Following the scan, the patient was monitored over 30 minutes for any complications. Barium esophagogram (single contrast) was done on the same day using 95% w/v barium suspension, and spot images were taken depending on the site of the lesion.
Processing
Thin section (0.6 mm) CT images were transferred to the workstation (Syngovia, Siemens) and loaded to the “CT colon” preset for obtaining virtual endoscopy (VE) and fly-through images. Volume rendered (VR) images were generated in solid and transparent modes. The time needed for image processing was noted. The images were evaluated by two experienced abdominal radiologists with 20 years and 7 years of experience.
Evaluation
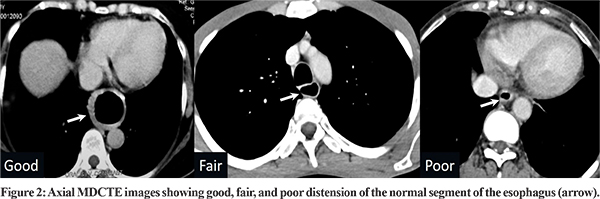
The amount of air injected during the procedure was noted. Patient comfort during the procedure was defined using a three-point scale: 1) No pain/discomfort; 2) Tolerable pain/discomfort; 3) Intolerable pain. The base images, virtual endoscopy, and volume-rendered images were reviewed by both radioloigsts independently. The degree of esophageal distension was classifed as ‘Good’ when the esophagus was well distended with thin esophageal wall proximal and distal to the lesion; ‘Fair’ when the esophagus was adequately distended without loss of diagnostic information; and ‘Poor’ when there was inadequate distension of the esophagus with loss of diagnostic details. The mucosal abnormality seen on conventional endoscopy and VE were categorized as ulcerative, polypoidal, and stricturing. The volume-rendered images were compared with barium esophagograms for the longitudinal extent of the disease. The overall diagnostic quality of MDCTE was finally classified as diagnostic or non-diagnostic. Descriptive statistics was used for demographic data and other parameters. Kappa weighted analysis was done to correlate concordance between MDCTE and conventional endoscopy and barium esophagogram. A p-value of 0.05 was considered significant. SPSS 17 (IBM, Chicago) was used for statistical evaluation.
Results
Seventy patients (44 males; 26 females) with confirmed esophageal carcinoma and a mean age of 55.1 years (range: 35-80 years) were included in the study. Dysphagia was present in all patients, with a mean duration of symptoms being 2.5 months (range: 1-12 months). The histology on endoscopic biopsy was squamous cell carcinoma in 55 patients (79%) and adenocarcinoma in 15 patients (21%).The most common site of the lesion was the middle third of the thoracic esophagus (28 patients; 40%). The frequency in other locations were 11% in upper third (n=8), 23% in lower third (n=16) and 26% at the gastroesophageal junction (n=18). On imaging, T4 disease was present in 28 (40%), T3 disease in 33 (47%), and T1/T2 disease in 9 (13%) patients. MDCTE was comfortable and tolerable in 68 patients (97%). In two patients, the procedure was intolerable due to the coiling of the NGT in the trachea causing respiratory distress (n=1), and abdominal distension due to excessive air insufflation (n=1). However, the procedure could be completed in both. There were no procedure-related complications, radiocontrast related allergy, or nephropathy in the study population. The mean amount of air injected during the procedure was 656 ml (range: 400-900 ml). Esophageal distension was good in 71.5% (n=50), fair in 18.5% (n=13) and poor in 10% (n=7) of patients (Figure 2).The average amount of air injected in patients with good, fair, and poor esophageal distension was 658 ml, 657 ml, and 655 ml, respectively, without significant difference between the groups (p=0.63). In the patients with poor distension, five tumors were in the mid-third of the esophagus, one in the upper third of the esophagus and one at the gastroesophageal junction. The reasons for poor distension were: misplaced NGT (n=4), fluid residue proximal to the obstruction (n=2) and uncooperative patient (n=1).
Initially, the average time for post-processing CT images was about 30 minutes, but with experience, it was reduced to 15 minutes during the second half of the study. The average esophageal wall thickness at the tumor location was 8 mm (range 3–40 mm), and tumor length was 71 mm (range 28–120 mm). Morphology on virtual and conventional endoscopy are shown in Table 1.
The overall quality of MDCTE was diagnostic in 63 (90%) patients (Figure 3). The volume-rendered images were equal or superior to barium esophagogram in defining the longitudinal extent of the disease in 90% (63/70) of patients (Figure 4). Kappa correlation between MDCTE and barium esophagogram for the length of the lesion was 0.913, and between MDCTE and conventional endoscopy for morphology was 0.630 (Figure 5).
Discussion
Patients with esophageal carcinoma have a poor prognosis because most patients have advanced disease at presentation.19 As resection is the only curative treatment option, early diagnosis and accurate staging are essential for devising a management plan. On routine CT scan, esophageal carcinoma is diagnosed based on abnormal wall thickening which depends on the extent of distension of the esophagus. This problem can be partly overcome by techniques like endoscopy, barium esophagogram, endoscopic ultrasonography (EUS), CT with oral contrast, magnetic resonance imaging with diffusion-weighted imaging, and Positron Emission Tomography (PET).20 Most of these methods are complementary. Barium esophagogram is highly sensitive, with a lesion detection rate of 98%, and positive predictive value of 42%.21 Although conventional endoscopy and barium studies can better demonstrate the mucosal extent of the lesion, they have limitations, including inaccurate assessment of the depth of tumor infiltration and the inability to contribute to disease staging.22,23 Also, endoscopy is invasive, and evaluation beyond a stricturing growth may not be possible due to difficulty passing the endoscope accross the malignant stricture.22 Endoscopic ultrasonography (EUS) better defines the longitudinal extent of the disease and is useful in local staging.24 The sensitivity of EUS in the preoperative determination of T and N stages in esophageal carcinoma is 92% and 85%, respectively.25 However, EUS cannot detect distant metastatic disease either in regional lymph nodes or solid organs due to a smaller field of view, is limited by the inability of the probe to cross the stricture, and carries a risk of perforation.20,22 MRI with T2-weighted and diffusion-weighted sequences have accuracies of 75–87% in staging esophageal carcinoma.23 However, routine use is difficult due to artifacts, cost, and long scan times.26 PET-CT is often used in the staging of esophageal carcinoma and is useful in detecting un-suspected distant metastasis.27 It may have a role in assessing response to neoadjuvant CRT and in evaluation for recurrent disease.28 However, due to high cost and limited availability, its routine use is restricted. Further, the absence of esophageal distension may not accurately define the extent of the tumor.12 MDCT and virtual endoscopy (VE) may become a single modality providing all the information needed preoperatively. Many authors have investigated it to evaluate benign and malignant esophageal conditions (Table 2).18 Proper gaseous distension of the esophageal lumen is the most critical factor in this technique. Optimal and consistent esophageal distension can be achieved by either actively insufflating air or carbondioxide into the esophagus or passively by using effervescent granules. Active distension requires the insertion of a tube, which increases procedure time and patient discomfort. Passively distending the esophagus requires much more patient co-operation. However, studies have shown good results with both techniques (Table 2).
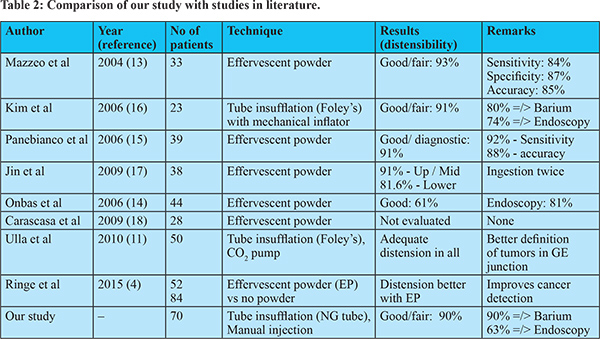
There are only eight studies of MDCTE in the English literature, most with less than 50 patients (Table 2). Six studies used effervescent powder for esophageal distension and obtained good or fair distension in more than 90% of patients. Jin et al.,17 suggested the ingestion of effervescent powder twice for better distension, although this may need more co-operation from the patient. Only two studies used a tube for insufflations; room air was used in one study and CO2 in the other.11,16 The results in these studies were also similar to studies using effervescent powder. Kim et al.16 compared MDCTE with conventional modalities and demonstrated that MDCTE is superior or equal to both barium esophagogram (in 80%) and endoscopy (in 74% of patients), similar to our findings. However, our study included the largest sample size thus far of 70 patients. We achieved good or fair esophageal distension in 90% of patients using manual insufflation of room-air. Ulla et al.,11 in their study of 50 cases using mechanical insufflators required 1000-1200 ml of CO2 gas under sustained pressure to achieve optimal distension in all cases. Although CO2 is inert and causes less patient discomfort, maintaining a CO2 cylinder and mechanical insufflator in a radiological suite may be difficult and expensive. We successfully used a lesser amount of room air without causing much patient discomfort. The average amount of air injected was not significantly different between patients with good or poor oesophageal distension. Patient co-operation is necessary for good oesophageal distension. Spraying of the posterior wall of the pharynx with a local anesthetic agent is helpful. Patients need to be explained about the procedure beforehand and appropriately instructed. Use of hypotonic agents like hyoscine butyl bromide 10-15 minutes before the procedure may be valuable in producing optimal distension.13 This procedure needs less co-operation by the patients than the use of effervescent agents, which causes passive esophageal distension.13 In addition to the esophagus, distension of the stomach is possible with tube insufflation and helps in defining the lower extent of the disease in gastroesophageal junction tumors.11 Many patients with oesophageal cancer have absolute dysphagia and have retained fluid in the esophageal lumen affecting the quality of VE images, which can be overcome by aspiration of the retained fluid prior to MDCTE. There were a few limitations to our study. First, we did not have a gold standard for comparison. No patient underwent surgery, and all patients received chemotherapy (neoadjuvant or palliative). Hence, the correlation at surgery was not possible. Second, we did not evaluate the diagnostic ability of MDCTE, as all patients had confirmed malignancy. Third, the images on VE do not have enough resolution to detect minute mucosal details, especially ulcerations. Therefore, it was often difficult to characterize the mucosal ulcerations confidently in a lesion. Further, obtaining biopsy and qualitative information (e.g., easy touch bleeding) is not possible with VE. Lastly, the post-processing of CT images requires special software, time, and expertise. In conclusion, MDCTE by tube insufflation of room air provides adequate esophageal distension in 90% of patients of esophageal carcinoma without causing significant discomfort. The results are better than barium esophagogram in most patients. Thus it can be a one-stop modality for accurate definition of the longitudinal extent of the disease as well as staging in patients of esophageal carcinoma.
References - Nandkumar A, Gangadharan P, Visweswara RN. Developement of an atlas of cancer in India. First all india report 2001 - 2002, National cancer registry program, ICMR. April, 2004.
- Smit JK, Güler S, Beukema JC, Mul VE, Burgerhof JG, Hospers GA, et al. Different recurrence pattern after neoadjuvant chemo-radiotherapy compared to surgery alone in esophageal cancer patients. Ann Surg Oncol 2013;20:4008-15.
- Oppedijk V, van der Gaast A, van Lanschot JJ, van Hagen P, van Os R, van Rij CM, et al. Patterns of recurrence after surgery alone versus preoperative chemo-radiotherapy and surgery in the CROSS trials. J Clin Oncol 2014;32:385-91.
- Ringe KI, Meyer S, Ringe BP, Winkler M, Wacker F, Raatschen HJ. Value of oral effervescent powder administration for multi-detector CT evaluation of esophageal cancer. Eur J Radiol 2015; 84:215-20.
- Ulla M, Gentile E, Yeyati EL, Diez ML, Cavadas D, Garcia-Monaco RD, et al. Pneumo-CT assessing response to neoadjuvant therapy in esophageal cancer: Imaging-pathological correlation. World J Gastrointest Oncol 2013; 5:222-9.
- Kim TJ, Kim HY, Lee KW, Kim MS. Multimodality assessment of esophageal cancer: preoperative staging and monitoring of response to therapy. Radiographics 2009;29:403–21.
- Swisher SG, Maish M, Erasmus JJ, Correa AM, Ajani JA, Bresalier R, et al. Utility of PET, CT, and EUS to identify pathologic responders in esophageal cancer. Ann Thorac Surg 2004; 78: 1152-60.
- Eloubeidi MA, Desmond R, Arguedas MR, Reed CE, Wilcox CM. Prognostic factors for the survival of patients with esophageal carcinoma in the U.S.: the importance of tumor length and lymph node status. Cancer 2002; 95: 1434-43.
- Griffiths EA, Brummell Z, Gorthi G, Pritchard SA, Welch IM. Tumor length as a prognostic factor in esophageal malignancy: univariate and multivariate survival analyses. J Surg Oncol 2006; 93: 258-67.
- Sillah K, Williams LR, Laasch HU, Saleem A, Watkins G, Pritchard SA, et al. Computed tomography overestimation of esophageal tumor length: Implications for radiotherapy planning. World J Gastrointest Oncol 2010;2:197-204.
- Ulla M, Cavadas D, Munoz I, Beskow A, Seehaus A, Garcia-Monaco R. Esophageal cancer: pneumo-64-MDCT. Abdom Imaging 2010; 35:383-9.
- Ulla M, Gentile EMJ, Cavadas D, Yeyati EL, Frank L, Argerich JI, et al. Esophageal cancer characterization with pneumo-64-MDCT. Abdom Imaging 2012; 37:501-11.
- Mazzeo S, Caramella D, Gennai A, Giusti P, Neri E, Melai L, et al. Multidetector CT and virtual endoscopy in the evaluation of the esophagus. Abdom Imaging 2004; 29:2-8.
- Onbas O, Eroglu A, Kantarci M, Polat P, Alper F, Karaoglanoglu N, et al. Preoperative staging of esophageal carcinoma with multi-detector CT and virtual endoscopy. Eur J Radiol 2006; 57:90-95.
- Panebianco V, Grazhdani H, Iafrate F, Petroni M, Anzidei M, Laghi A, et al. 3D CT protocol in the assessment of the esophageal neoplastic lesions: can it improve TNM staging? Eur Radiol 2006; 16:414-21.
- Kim SH, Lee JM, Han JK, Kim YH, Lee JY, Lee HJ, et al. Three-dimensional MDCT imaging and esophagography for evaluation of esophageal tumors: preliminary study. Eur Radiol 2006; 16:2418-26.
- Jin GY, Park SH, Han YM. Usefulness of MDCT evaluation of the intraluminal surface of esophageal masses using only effervescent powder without injection of hypotonic agent. Abdom Imaging 2009; 34:424-9.
- Carrascosa P, Capunay C, Lopez EM, Salis G, Mazzadi S, Carrascosa J. Esophageal stenosis: three-dimensional multi-detector CT and virtual endoscopy. Abdom Imaging 2009; 34:19-25.
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003; 349:2241–52.
- Tirumani H, Rosenthal MH, Tirumani SH, Shinagare AB, Krajewski KM, Ramaiya NH. Esophageal carcinoma: current concepts in the role of imaging in staging and management. Canadian Assoc Radiol J 2015; 66:130-9.
- Levine MS, Chu P, Furth EE, Rubesin SE, Laufer I, Herlinger H. Carcinoma of the esophagus and esophagogastric junction: sensitivity of radiographic diagnosis. Am J Roentgenol 1997; 168:1423–6.
- Kumbasar B. Carcinoma of esophagus: radiologic diagnosis and staging. Eur J Radiol 2002; 42:170–80.
- Wu LF, Wang BZ, Feng JL, Cheng WR, Liu GR, Xu XH, et al. Preoperative TN staging of esophageal cancer: comparison of miniprobe ultrasonography, spiral CT and MRI. World J Gastroenterol 2003; 9:219–24.
- Maluf-Filho F, Dotti CM, Halwan B, Queiros AF, KupskiC, Chaves DM, et al. An evidence-based consensus statement on the role and application of endosonography in clinical practice. Endoscopy 2009;41:979-87.
- Puli SR, Reddy JB, Bechtold ML, Antillon D, Ibdah JA, Antillon MR. Staging accuracy of esophageal cancer by endoscopic ultrasound: A meta-analysis and systematic review. World J Gastroenterol 2008; 14:1479-90.
- van Rossum PSN, van Lier ALHMW, Lips IM, Meijer GJ, Reerink O, van Vulpen M, et al. Imaging of esophageal cancer with FDG-PET/CT and MRI. Clin Radiol 2015; 70:81-95.
- van Westreenen HL, Heeren PA, van Dullemen HM, van der Jagt EJ, Jager PL, Groen H, et al. Positron emission tomography with F-18-fluorodeoxyglucose in a combined staging strategy of esophageal cancer prevents unnecessary surgical explorations. J Gastrointest Surg 2005;9:54-61.
- Chowdhury FU, Bradley KM, Gleeson FV. The role of 18F-FDG PET/CT in the evaluation of oesophageal carcinoma. Clin Radiol 2008;63:1297-1309.
|