Introduction
Cryptogenic cirrhosis (CC) means cirrhosis of liver of undetermined etiology. It is a diagnosis of exclusion and the diagnosis should be made only after extensive evaluation to exclude recognizable etiologies. The proposed etiologies of CC include non-alcoholic steatohepatitis (NASH), occult alcohol abuse, occult viral hepatitis, autoimmune hepatitis, occult biliary disease, hepatic vascular disease, and celiac disease among other rare causes.
Older studies found the prevalence of CC to be 5-30% among patients with cirrhosis1,2. Subsequently with the observation that NASH patients and many CC patients shared similar characteristics such as high prevalence of obesity and diabetes, NASH was deemed to be the most common cause of CC3,4.
A landmark study by Caldwell et al.3 had observed that more than half of CC patients are female. The average age of CC patients is around 60 years3,4. These patients are almost a decade older than patients with other etiologies of cirrhosis3. Most of CC patients presented with complications of portal hypertension i.e. with advanced disease3. Both diabetes and obesity are more prevalent in CC patients in comparison to cirrhotic patients with primary biliary cholangitis (PBC) or hepatitis C3,4. On the other hand, the prevalence of obesity and diabetes is remarkably similar to NASH patients3. Thus, these studies3,4 concluded that NASH plays an under-recognized role in many patients with CC.
The prevalence of true CC (where cause is truly unknown) is coming down with increasing diagnosis of NASH in the last decade. United Network for Organ Sharing (UNOS) database for liver transplant in the US for the period of 2002-2016 suggests that diagnosis of NASH increased from 1% to 16% while CC declined from 8% to 4%5.
NASH is often asymptomatic in early stages. It can progress silently to cirrhosis with loss of classical histological features6 and may present for the first time with cirrhosis or its complications masquerading as CC. Non-alcoholic fatty liver disease (NAFLD) has found to be associated with obesity7, diabetes8, dyslipidemia8 and insulin resistance9 which are the main features of the metabolic syndrome9.
Thus, there is a need to study these risk factors of NAFLD in CC patients to characterize their role in causation of the disease and provide evidence of antecedent NAFLD. Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) is a good surrogate marker for insulin sensitivity and has been found to be raised in NAFLD10. Adiponectin is an adipokine which improves insulin sensitivity11. Adiponectin is reduced in NAFLD and further decreases with progression to NASH and fibrosis11. Hence, HOMA-IR and serum A diponectin may be useful in corroborating association with NAFLD in CC patients.
The aim of the study was to compare the prevalence of risk factors for NAFLD in patients of cryptogenic cirrhosis and cirrhosis due to other etiologies (excluding patients with NASH). Biomarkers HOMA-IR and serum adiponectin were also assessed in both the groups.
Methods
This was a retrospective, case-control observational study conducted in a tertiary care hospital in the Department of Gastroenterology between December 2017- March 2019. The study was conducted in consecutive patients of liver cirrhosis.
Inclusion criteria:
* Patients having cirrhosis of liver on the basis of imaging findings (ultrasound abdomen revealing liver having coarse echotexture/ irregular outline/ increased ultrasound attenuation/ hypertrophy of the caudate lobe or left lobe with or without signs of portal hypertension- ascites/ splenomegaly/collaterals).
* Patients enrolled were within 18-70 yrs of age.
Exclusion Criteria:
* Proven NASH-related cirrhosis
* Coexisting hepatocellular carcinoma (HCC)
Cases: Patients with cryptogenic cirrhosis (after exclusion of established causes of liver cirrhosis)
Controls: Patients with cirrhosis due to known causes (excluding NASH).
The purpose of the study was explained to the subjects and written informed consent was obtained from eligible subjects who agreed to participate. A detailed history was obtained including gender, age at diagnosis of cirrhosis, history/duration of obesity/hypertension/ diabetes/ dyslipidemia, history of alcohol or intravenous drugs, blood transfusions, potential occupational exposure to hepatotoxins, family history of liver disease, and family or personal history of autoimmune diseases. A thorough examination including anthropometry was performed.
Investigations done for etiology of liver cirrhosis:
* Hepatitis B screening (hepatitis B surface antigen, Total anticore antibody, HBV DNA)
* Hepatitis C screening (anti-HCV, HCV RNA )
* Autoimmune etiology antinuclear antibody (ANA) titers/ Anti smooth muscle antibody (ASMA), Serum Immunoglobulin levels
* Serum Ceruloplasmin
* Iron studies
* Antimitochondrial antibody
* Liver biopsy (if clinically indicated)
The following risk factors for NAFLD were screened in all patients:
1) Obesity: Obesity was defined according to Asian Indian guidelines12 BMI= Weight (kg)/ (height in metres)2 Normal BMI: 18.0-22.9 kg/m2, Overweight: 23.0-24.9 kg/m2, Obesity: >25 kg/m2.
The average body weight in past 10 years was used as the criteria for calculating BMI.
For patients with ascites/ pedal edema, dry weight was calculated by subtracting a percentage of weight based upon the severity of ascites (mild 5%; moderate 10%; severe 15%), with an additional 5% subtracted if bilateral pedal oedema was present. The dry-weight BMI was then calculated by dividing the patient’s estimated dry weight (kg) by the square of the patient’s height (m).
2) Diabetes: History of diabetes mellitus in the past 10 years (fasting glucose = 126 mg/dL orHbA1c = 6.5%) requiring dietary management, oral hypoglycemic agents or insulin.
3) Dyslipidemia: Dyslipidemia was based on a previous diagnosis of dyslipidemia in past 10 years (triglycerides = 150 mg/dL, and/or serum total cholesterol = 200 mg/dL, and/or HDL < 50 mg/dL in women and < 40 mg/dL in men), and/or use of lipid-lowering drugs.
4) Hypertension: Previous diagnosis of hypertension in past 10 years (blood pressure = 130/85 mmHg) and/or on anti-hypertensive medications
5) Metabolic syndrome: Metabolic syndrome was defined according to the Asian Indian guidelines12; any 3 of 5 of the following factors: triglycerides 150 mg/dL or greater, high-density lipoprotein-cholesterol of less than 40 mg/dL in men and less than 50 mg/dL in women, dysglycemia (fasting glucose of 100 mg/dL or greater), abdominal obesity (waist circumference for males: >90 cm, females: > 80 cm), and hypertension (systolic blood pressure of 130 mmHg or greater or diastolic blood pressure of 85 mmHg or greater).
Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) was calculated according to the following formula:
Score = (Fasting insulin in mIU/L)*(Fasting glucose in mg/dL ) / 405.
Serum Adiponectin levels were measured once using RayBio Human Acrp30ELISA Kit manufactured by RayBiotech.
Statistical Analysis
For the purpose of calculation of sample size the primary risk factor of interest was taken as obesity in the 2 groups that we proposed to study. Tellez-Avila et al.14 reported that 16.4 % of CC and 8.2 % of non-CC patients were obese in their study. To be able to detect a difference of 10% with power of 80% and significance level 5%, the sample size came to 50 patients in each group.
Statistical testing were conducted with the statistical package for the social science system version SPSS 17.0. The comparison of normally distributed continuous variables between the groups were performed using Student’s t test. Nominal categorical data between the groups were compared using Chi-squared test or Fisher’s exact test as appropriate. Non-normal distribution continuous variables were compared using Mann Whitney U test. Multivariable Logistic regression analysis was done. For all statistical tests, a p value less than 0.05 was taken to indicate a significant difference. The protocol of the study was approved by the ethical committee of the institution. The data collected has been kept confidential.
Results
A total of 152 patients (consecutive liver cirrhosis patients) were screened for the study. Patients with proven NASH (n=22), insufficient data (n=8), coexistent HCC (n=10) and lack of consent (n=12) were excluded from the study. Liver biopsy was done in 13 patients (autoimmune hepatitis= 3, PBC=1, celiac disease=1, HBV= 2, alcoholic liver disease=1, CC=5). Among them 5 biopsies had insufficient criteria to ascertain any etiology and were hence allotted to the case group.
In this study, etiology of cirrhosis in the control group were alcoholic liver disease (54%, n=27), hepatitis B (24%, n=12), hepatitis C (12%, n=6), autoimmune (6%, n=3), PBC (2%, n=1) and celiac disease (2%, n=1). Majority of CC patients were Child Pugh A (42%) followed by Child-Pugh B (40%). On the other hand, patients in the control group were primarily Child-Pugh B (60%) followed by Child-Pugh C (34%). Mean MELD score was also higher in control group as compared to cases.
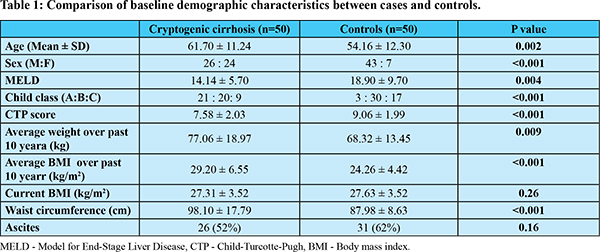
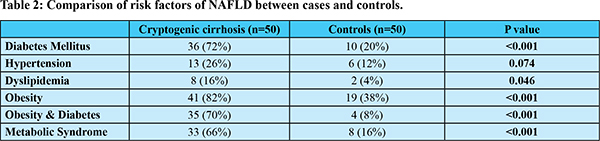
Baseline characteristics of CC and controls have been compared in Table 1. Mean age of CC patients (61.70 ± 11.24 years) was significantly higher than control group. Proportion of females was significantly higher in CC group as compared to controls (48% vs 14%). Average weight and BMI over past 10 years was significantly higher in CC group. Table 2 summarizes the comparison of risk factors of NAFLD. Proportion of patients with history of obesity was higher in CC in comparison with controls (82% vs 38%; p<0.001). CC patients had a significantly higher proportion of diabetics than controls (72% vs 20%; p<0.001). History of dyslipidemia was significantly higher in CC patients than controls (16% vs 4%; p=0.046). Prevalence of metabolic syndrome was significantly higher in CC patients compared to controls (66% vs 16%; p<0.001).
Comparison of metabolic biomarkers is summarized in Table 3. Fasting insulin levels were significantly higher in CC group than controls (p=0.006). Insulin resistance (HOMA-IR) was significantly higher in CC group (2.72 ± 2.26) as compared to controls (1.48 ± 1.28)(p=0.003). Fasting Adiponectin levels were significantly lower in CC group (11.80 ± 11.18 µg/ml) than in controls (18.06 ± 15.72 µg/ml) (p=0.024). On multivariable logistic regression analysis (Table 4) after adjusting for age and sex, previously diagnosed obesity and higher HbA1c were independently associated with CC.
Discussion
In the past two decades, the proportion of CC has come down significantly after many cases have been attributed to NASH. However, antecedent NAFLD in CC is difficult to prove as characteristic imaging and histological changes in NAFLD tend to disappear with progression to fibrosis and cirrhosis13. Therefore, studies have resorted to detecting indirect evidence of pre-existing NASH in the form of obesity, diabetes and metabolic syndrome3,4,14. After the onset of cirrhosis, metabolic changes in the body may lead to alteration of these parameters. The onset of edema and ascites in advanced cirrhosis may lead to erroneous anthropometric measurements. Hence, past history of these comorbidities must be taken into account to prevent fallacious results. In this study, history of hypertension, diabetes, dyslipidemia, obesity and metabolic syndrome in the past 10 years were taken into account.
As in our study, previous studies had also observed that CC patients had higher mean/median age than virus, alcohol and other etiologies of cirrhosis3,15,16. The average age of CC patients is higher than NASH cirrhosis17, which may suggest that CC represents burnt out NASH as an advanced stage of the same underlying disease. Early stages of disease may be asymptomatic and patients frequently present late with complications of cirrhosis.
Males and females contributed almost an equal proportion of CC patients (52% vs 48%) in this study. Older studies had reported a predominance of female patients in CC (~70%) as compared to controls (25%-56%)3,14-16,18. However recent studies by Younossi et al. (2018)19 and Thuluvath et al.(2018)16 have observed an almost equal proportion of males and females in CC as well as NASH patients corresponding with our findings. Our study corroborates this changing demographic pattern for CC and NASH cirrhosis.
South Asians have a certain unique body composition known as “Asian Indian phenotype”. They tend to have higher central adiposity at lower BMIs, higher insulin resistance and increased prevalence of diabetes20. Hence, proposed guidelines for Asian Indians12 were used in this study to quantify obesity i.e. BMI: >25 kg/m2. CC patients had a mean BMI of 29.2 ± 6.55 kg/m2 over past 10 years in this study. More CC patients had history of obesity in this study (82%) as compared to western studies (42% to 69%)3,4,16,18. Duseja et al21 from India also observed that the mean BMI was significantly higher in patients with CC (26.06 ± 5.96 kg/m2) versus patients with virus-related cirrhosis (22.12 ± 1.71 kg/m2). The high prevalence of obesity in CC patients in our study may reflect a higher proportion of NASH among cryptogenic cirrhosis in India due to the unique metabolic phenotype of Asian Indians. Also majority of previous studies had not included past history of obesity which may have led to underestimation of obesity in these patients.
This study found that 72% of CC patients were diabetic in comparison to 20% in controls (p<0.001). Previous Western, Asian and Indian studies had also observed prevalence of diabetes ranging from 40% to 70% in CC which is significantly higher as compared to cirrhosis of other known etiologies3,4,14-16,18,21-23. The prevalence of metabolic syndrome, obesity and diabetes has risen exponentially in the past 2 decades due to rapid urbanization and lifestyle changes. The high prevalence of diabetics in CC in this study parallels the rise in NASH related cirrhosis5 in recent times.
Indians are more prone than the Western population for developing metabolic syndrome (MS) and it is estimated that one third of the urban Indian population has MS24. This study showed a significantly higher incidence of MS in CC patients (66%) vs. control group (16%) (p<0.001). Previous studies from Mexico14 and Italy18 also revealed 29.1% and 52.9% prevalence of MS in CC patients.
Various other studies from Asia15,25 and India21-23 showed similar results. Kojima et al.25 found higher prevalence of obesity (54% vs 20%) and diabetes (54% vs 26%) in CC vs controls. Our CC group had a higher prevalence of obesity (82%) and diabetes (72%) than the Japanese studies15,25 probably signifying the unique Asian Indian phenotype which exists in India.
Insulin resistance (IR) is a key pathogenic factor in NAFLD. HOMA-IR which is a surrogate marker for IR was significantly higher in CC group (2.72 ± 2.26) as compared to controls (1.48 ± 1.28) (p=0.003) in this study. Previous studies by Kojima et al.25 and Deet al.26 had also reported higher insulin resistance in CC as compared to cirrhosis of other etiologies. Bugianesi et al.10 observed that HOMA-IR> 2.7 was an independent predictor of advanced fibrosis in NAFLD and our CC patients fit into that category. Hyperinsulinemic state and insulin resistance may also be part of the pathophysiology of cirrhosis due to hepatic parenchymal cell damage and portal-systemic shunting ultimately culminating into hepatogenous diabetes. However, higher IR in the CC group than controls suggests the possibility of underlying NAFLD.
Previous studies have proved that NAFLD is associated with low adiponectin levels11. A diponectin levels inversely correlate to steatosis and necroinflammation in NASH, but the relationship with fibrosis is less clear27. On the other hand, adiponectin may also be raised in all cirrhotics irrespective of etiology28.Studies had found that in cirrhotics, mean serum adiponectin ranged from 15.2- 21.59 µg/mL with higher levels as disease progresses28. An Indian study showed that CC patients (10.2 ± 5.8 µg/ml) had higher adiponectin as compared to NAFLD/ NASH patients29. However, there are various serum adiponectin assays available in the market which have not been standardized, leading to lack of comparability among various studies. There is also alack of normal reference ranges for the assay leading to limited clinical utility. In this study, CC patients had significantly low adiponectin levels (11.80 ± 11.18 µg/ml) than cirrhosis of known etiology (18.06 ± 15.72µg/ml) (p=0.024). This reflects that pathogenesis of cirrhosis in CC patients may be related to NAFLD.
Multivariable logistic regression analysis revealed that previously reported obesity and high HbA1c were independently associated with CC. Kojima et al.25 had a similar observation that elevated hemoglobin A1c, BMI = 25 kg/m2, and normal aminotransferase levels were independent predictors of CC. This finding suggests that obesity and diabetes are independently associated with CC and provides further evidence of antecedent NASH.
In the CC group, 5 patients underwent liver biopsies and had insufficient criteria to ascertain any etiology. In 3 out of these 5 biopsies there were some foci of macrosteatosis/cellular ballooning/glycogenated nuclei which may suggest NASH as the probable etiology.
Indian studies have also shown that 85% of cryptogenic HCC had at least one risk factor for NAFLD30 and they have higher BMI with a higher prevalence of type 2 diabetes mellitus as compared virus related HCC21.
This study has a few limitations. Case and control groups were not matched for age, sex and severity of liver disease which may have led to selection bias. However, the past history of various risk factors (past 10 years) of NAFLD were taken into account in this study which is unlikely to be affected due to our unmatched control group. There may have been a recall bias due to the retrospective nature of the study. Few risk factors may have been confounded by the presence of cirrhosis.
Conclusion
Cryptogenic cirrhosis patients are significantly older with higher proportion of females than cirrhosis of other etiologies. The prevalence of obesity, diabetes, dyslipidemia and metabolic syndrome is higher in cryptogenic cirrhosis than in cirrhosis due to other etiologies. Insulin resistance is higher and adiponectin levels are lower in cryptogenic cirrhosis patients than cirrhosis due to other etiologies. Previously reported obesity and high HbA1c are independently associated with cryptogenic cirrhosis. All these findings suggest that NAFLD/NASH may be an important etiology of cryptogenic cirrhosis in this cohort.
References
- Saunders JB, Walters JR, Davies AP, Paton A. A 20-year prospective study of cirrhosis. Br Med J (Clin Res Ed). 1981 Jan 24;282(6260):263-6.
- Kodali VP, Gordon SC, Silverman AL, McCray DG. Cryptogenic liver disease in the United States: further evidence for non-A, non-B, and non-C hepatitis. Am J Gastroenterol. 1994;89(10):1836-9.
- Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29(3):664-9.
- Poonawala A, Nair SP, Thuluvath PJ. Prevalence of obesity and diabetes in patients with cryptogenic cirrhosis: a case-control study. Hepatology. 2000;32(4):689-92.
- Thuluvath PJ, Hanish S, Savva Y. Liver Transplantation in Cryptogenic Cirrhosis: Outcome Comparisons Between NASH, Alcoholic, and AIH Cirrhosis. Transplantation. 2018;102(4):656-663.
- Lee RG. Nonalcoholic steatohepatitis: a study of 49 patients. Hum Pathol. 1989;20(6):594-8.
- Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12(5):1106-10.
- Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11(1):74-80.
- Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107(5):450-5.
- Bugianesi E, Marchesini G, Gentilcore E, Cua IH, Vanni E, Rizzetto M, et al. Fibrosis in genotype 3 chronic hepatitis C and nonalcoholic fatty liver disease: Role of insulin resistance and hepatic steatosis. Hepatology. 2006;44(6):1648-55
- Polyzos SA, Toulis KA, Goulis DG, Zavos C, Kountouras J. Serum total adiponectin in nonalcoholic fatty liver disease: a systematic review and meta-analysis. Metabolism. 2011;60(3):313-26.
- Misra A, Chowbey P, Makkar BM, Vikram NK, Wasir JS, Chadha D, et al; Concensus Group. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India. 2009;57:163-70.
- Caldwell S. Cryptogenic cirrhosis: what are we missing? Curr Gastroenterol Rep. 2010;12(1):40-8.
- Tellez-Avila FI, Sanchez-Avila F, García-Saenz-de-Sicilia M, Chavez-Tapia NC, Franco-Guzman AM, Lopez-Arce G, et al. Prevalence of metabolic syndrome, obesity and diabetes type 2 in cryptogenic cirrhosis. World J Gastroenterol. 2008;14(30):4771-5.
- Sakugawa H, Nakasone H, Nakayoshi T, Kawakami Y, Yamashiro T, Maeshiro T, et al. Clinical characteristics of patients with cryptogenic liver cirrhosis in Okinawa, Japan. Hepatogastroenterology. 2003;50(54):2005-8.
- Thuluvath PJ, Kantsevoy S, Thuluvath AJ, Savva Y. Is cryptogenic cirrhosis different from NASH cirrhosis? J Hepatol. 2018;68(3):519-525.
- Argo CK, Caldwell SH. Epidemiology and natural history of nonalcoholic steatohepatitis. Clin Liver Dis. 2009;13(4):511-31.
- Rinaldi L, Nascimbeni F, Giordano M, Masetti C, Guerrera B, Amelia A, et al. Clinical features and natural history of cryptogenic cirrhosis compared to hepatitis C virus-related cirrhosis. World J Gastroenterol. 2017;23(8):1458–1468.
- Younossi Z, Stepanova M, Sanyal AJ, Harrison SA, Ratziu V, Abdelmalek MF, et al. The conundrum of cryptogenic cirrhosis: Adverse outcomes without treatment options. J Hepatol. 2018;69(6):1365-1370.
- Deepa R, Sandeep S, Mohan V. Abdominal obesity, visceral fat and type 2 diabetes- Asian Indian phenotype. In: Mohan V, Rao GHR, editors. Type 2 diabetes in South Asians: epidemiology, risk factors and prevention. New Delhi, India: Jaypee Brothers Medical Publishers (P) Ltd; 2006. pp. 138–52.
- Duseja A, Sharma B, Kumar A, Kapil S, Das A, Dhiman RK et al. Nonalcoholic fatty liver in a developing country is responsible for significant liver disease. Hepatology. 2010;52(6):2248-2249.
- Amarapurkar D, Das HS. Chronic liver disease in diabetes mellitus. Trop Gastroenterol. 2002;23(1):3-5.
- Duseja A, Nanda M, Das A, Das R, Bhansali A, Chawla Y. Prevalence of obesity, diabetes mellitus and hyperlipidaemia in patients with cryptogenic liver cirrhosis. Trop Gastroenterol. 2004;25(1):15-7.
- Pandit K, Goswami S, Ghosh S, Mukhopadhyay P, Chowdhury S. Metabolic syndrome in South Asians. Indian J Endocrinol Metab. 2012;16(1):44–55.
- Kojima H, Sakurai S, Matsumura M, Umemoto N, Uemura M, Morimoto H, et al. Cryptogenic cirrhosis in the region where obesity is not prevalent. World J Gastroenterol. 2006;12(13):2080-5.
- De BK, Mani S, Mandal SK, Mondal SS, Bhattacharya R, Pramanik AB, et al. Cryptogenic cirrhosis: metabolic liver disease due to insulin resistance. Indian J Med Sci. 2010;64(11):508-19.
- Van der Poorten D, Samer CF, Ramezani-Moghadam M, Coulter S, Kacevska M, Schrijnders D, et al. Hepatic fat loss in advanced nonalcoholic steatohepatitis: are alterations in serum adiponectin the cause? Hepatology. 2013 Jun;57(6):2180-8.
- Da Silva TE, Costa-Silva M, Correa CG, Denardin G, Alencar MLA, Coelho MSPH, et al. Clinical Significance of Serum Adiponectin and Resistin Levels in Liver Cirrhosis. Ann Hepatol. 2018;17(2):286-299.
- Sanal M, Sarin S. Serum adipokine profile in Indian men with nonalcoholic steatohepatitis: Serum adiponectin is paradoxically decreased in lean vs. obese patients. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2009;3(4):198-203.
- David D, Raghavendran A, Goel A, Bharath Kumar C, Kodiatte TA, Burad D et al. Risk factors for non-alcoholic fatty liver disease are common in patients with non-B non-C hepatocellular carcinoma in India. Indian J Gastroenterol. 2017;36(5):373-379.