|
Amit Kumar Dutta, John Titus George, Sumanth Koppolu Department of Gastroenterology, Christian Medical College and Hospital, Vellore, Tamil Nadu, India.
Corresponding Author:
Prof. Amit Kumar Dutta Email: akdutta1995@gmail.com
Abstract
Small intestinal bacterial overgrowth (SIBO) denotes an increase in the concentration of bacterial population in small intestine. There are several mechanisms such as acid secretion in the stomach, motility of gastrointestinal tract and immune response which prevent overgrowth of bacteria in healthy individuals. Breakdown of this mechanism predisposes to the development of SIBO. Conditions associated with increased risk of SIBO include diabetes mellitus, intestinal strictures and blind loops, post gastrectomy status, gastrointestinal dysmotility, chronic opioid or proton pump inhibitor use, immune deficiency states, etc. Usual symptoms of SIBO are non-specific and include bloating, diarrhoea and flatulence. Quantitative culture of duodenal/jejunal aspirate is the gold standard test for diagnosing SIBO. Breath tests are alternative diagnostic tools and are more frequently used in clinical practice due to their simplicity and non-invasive nature but are not very accurate. Recent developments such as availability of next generation sequencing and capsule breath test devices to diagnose SIBO appear promising for clinical application in future. Treatment of SIBO includes a short course of antibiotics, correction of nutritional deficiencies and treatment of predisposing factors. This review discusses the risk factors and clinical features of SIBO and provides an update on the diagnostic tests and management.
|
Introduction
Bacteria are present across the length of the gastrointestinal tract with the highest concentration noted in colon (1010-11/ml)1,2. The concentration is lower in stomach and jejunum (<103/ml) compared to ileum (104-6/ml). Normally, colon has more anaerobes (e.g., Bacteroidetes, Clostridium) and gram negative bacteria (e.g., Escherichia, Klebsiella, Enterobacter, Proteus, Acinetobacter, etc.) compared to the small intestine3-7. In the small intestine, gram positive organisms (Streptococcus, Staphylococcus, Lactobacillus, Enterococcus, etc.) are frequent and gram negative coliforms are less common4. Small intestinal bacterial overgrowth (SIBO), as the name suggests, denotes the presence of higher than expected concentration of bacteria in small intestine. With improvement in our knowledge of gut microbiota, newer insights are emerging on SIBO, yet several questions that loomed earlier still persist - Can symptoms be confidently attributed to SIBO? Does SIBO represent overgrowth of locally present bacteria or migrated bacterial population from distal gut? What should be the diagnostic criteria? What should be the best diagnostic strategy?8,3 Hence, there is risk of both underdiagnosing and over diagnosing SIBO in clinical practice3. In this paper we provide an overview of the predisposing factors, clinical features and current diagnostic and management strategy of this clinically challenging condition.
Definition of SIBO
The North American consensus defines SIBO as bacterial load greater than 103 colony forming unit(CFU)/ml which is the generally accepted criteria currently9,10. Some define SIBO based on presence of excess number of coliforms (>103 CFU/ml) while others have kept a higher cut-off (>105 CFU/ml) of bacterial population to define SIBO11,5. In clinical practice, breath tests are done more commonly than culture to diagnose SIBO. According to the North American consensus, a >20 part per million (PPM) increase of hydrogen from baseline or a >10PPM increase of methane from baseline after lactulose administration is considered positive for diagnosis of SIBO10. These definitions are based on studies usually done on small populations and may not be generalizable for different geographical locations owing to the variations in lifestyle and dietary habits. There are interindividual variations in the type and density of bacterial population in proximal small intestine. Also, the organisms increased in abundance may differ depending on the cause of SIBO, with obstruction and dysmotility favoring growth of gram negative organism and reduced acid secretion in stomach favoring gram positive organisms5,12.
Predisposing Factors for SIBO
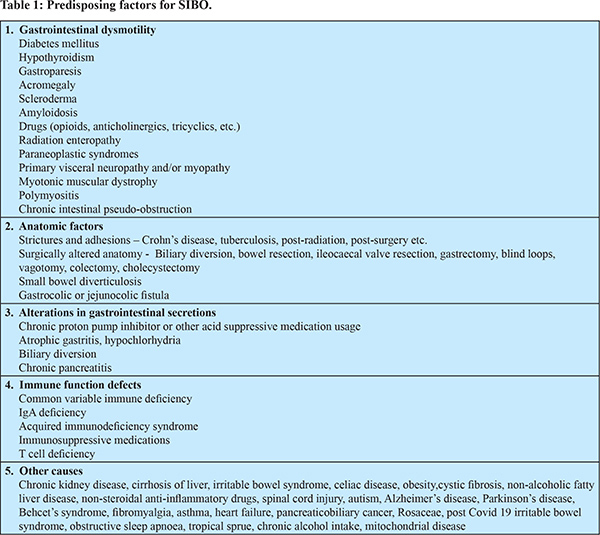
A number of factors influence the concentration and composition of bacteria in intestine13. The acidic secretion from stomach and other intestinal secretions, gastrointestinal motility, local immune response, intact ileocaecal valve, etc. keeps the small intestinal bacterial population in check. Alterations in these defense mechanisms predispose to SIBO, although in some conditions such as functional gastrointestinal disorders, the exact reason is not well known. Table 1 shows the various conditions associated with SIBO and Figure 1 shows the prevalence of SIBO in few selected conditions.Although the disorders are placed in separate categories for simplicity, in several of them, multiple factors may be responsible for causing SIBO. It is also important to note that the studies on prevalence of SIBO are affected by non-standardization of the definition and variability in the diagnostic methods chosen.
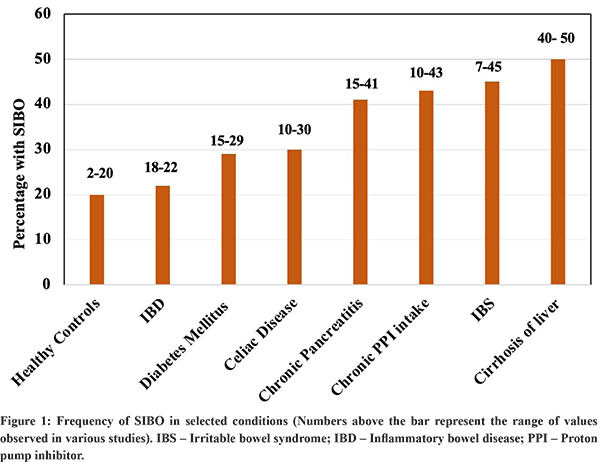
i) Subjects without organic disorders and FGIDs: SIBO has been noted in 2% to 20% of healthy individuals with higher prevalence in older age6,14,15. Dysbiosis is emerging as one of the contributory factors in several FGIDs (Functional gastrointestinal disorders or disorders of gut brain interaction)16. Among FGIDs, SIBO has been extensively studied in patients with irritable bowel syndrome (IBS). A meta-analysis of 50 studies on IBS, showed a pooled prevalence of SIBOto be 38% (95% CI, 32-44)17. Notably, the prevalence was higher (40%) when the diagnosis was made by breath test compared to culture (19%). The risk of SIBO is more in patients with IBS-diarrhoea subtype, women, those with symptoms of bloating and in older age subjects17,18,19. Three studies from India have found the frequency of SIBO in IBS to be 8.5%, 11% and 46%19-21. Another FGID where SIBO is commoners than control is dyspepsia. A meta-analysis of seven studies showed a higher risk of SIBO with functional dyspepsia compared to controls22. The odds ratio of SIBO in functional dyspepsia was 4.3 (95% CI, 1.1-17.5) although the quality of data was low.
ii) Altered gastrointestinal secretion: Gastric acid and pancreatic enzymes make the micro-environment less suitable for bacterial growth23,7. Most of the bacteria cannot survive in the stomach because of its highly acidic pH. Pancreatic enzymes degrade the proteins required for bacterial growth and digests the nutrients leading to absorption, thereby reducing stasis of nutrients24. Bile acids, with their detergent action on bacterial cell membrane and anti-microbial properties prevent bacterial overgrowth in proximal small intestine25,26,23,7. Conjugated Bile acids have an indirect protective action in the distal small bowel by activating Farsenoid X receptor pathway. This pathway upregulates the genes involved in producing antimicrobial substances and strengthening epithelial integrity26. Accordingly reduced gastric acid secretion, pancreatic enzyme insufficiency and chronic liver disease have all been associated with SIBO. Acid secretion is reduced in patients with achlorhydria, gastrectomy and those on proton pump inhibitors (PPIs). A meta-analysis of 19 studies with 7055 subjects showed a higher risk of SIBO with the use of PPI (OR 1.71, 1.2-2.43)27. In addition to SIBO, use of PPI has been associated with increased risk of gastrointestinal infections like C difficile, Salmonella and Campylobacter28. In cirrhosis, the decreased bile acid level may partially contribute to SIBO29,30. A study from North India showed the prevalence of SIBO to be around 49% in cirrhosis compared to 8% in controls (p=0.01) and the frequency increased with increasing disease severity31. A meta-analysis of 21 studies found the prevalence of SIBO to be 40.8% (95% CI, 34.8-47.1) in cirrhotics compared to 10.7% (5.7-19) in controls32. The frequency was higher in the presence of decompensated disease (50.5% vs. 31.2%; p < 0.001). Presence of SIBO was associated with a significantly higher rate of minimal hepatic encephalopathy, ascites and spontaneous bacterial peritonitis. SIBO has been reported in about 15% to 40% of patients with chronic pancreatitis33-35. The risk is higher in the presence of severe disease, use of opioids, low zinc levels and presence of diabetes mellitus33. In a study from South India in 48 patients with newly diagnosed chronic pancreatitis, SIBO was detected in 37.5% based on jejunal aspirate quantitative culture36. In a meta-analysis of 13 studies in patients with chronic pancreatitis, pooled prevalence of SIBO was 38.6% (95% CI, 25.5-53.5)37. Along with reduced pancreatic enzymes,dysmotility from usage of opioids and diabetes mellitus may also contribute to the overgrowth of bacteria in chronic pancreatitis38,39.
iii) Gastrointestinal dysmotility: The peristaltic waves of small intestine prevent stasis of chyme and growth of bacterial colonies in normal individuals3. Decreased activity of migratory motor complexes (MMC) has been associated with SIBO40. Dysmotility results from abnormal muscle and/or nerve function and can be transient (e.g. prolonged hospitalised states, medications, etc.) or chronic. Examples of condition where SIBO is associated with altered motility includes gastroparesis, chronic intestinal pseudo-obstruction, diabetes mellitus, hypothyroidism, scleroderma, amyloidosis, chronic kidney disease, dyselectrolytemias, ileus and drugs41,42. In a meta-analysis of 14 studies (1417 patients), the prevalence of SIBO in diabetes mellitus was 29% (95% CI, 20-39)43. Systemic sclerosis is an autoimmune disorder which affects different organs and gastrointestinal involvement is frequent. Dysmotility of small intestine due to muscle atrophy and fibrosis predisposes to SIBO and is associated with increased morbidity and mortality. The risk of SIBO was noted to be ten times higher in systemic sclerosis compared to controls in a meta-analysis of 28 studies with 1112 patients and 335 controls44. The pooled prevalence of SIBO in systemic sclerosis was 39.9% (95% CI, 33.1-47.1). Among patients with gastroparesis, pooled prevalence of SIBO has been reported to be 41% (95% CI, 23-58)45.
iv) Anatomical factors: Anatomical alterations in small bowel (post-operative, disease related, etc.) can lead to bacterial overgrowth by promoting stasis of luminal contents. Examples include blind loops, diverticula, adhesions and strictures. Ileocaecal (IC) valve is an important anatomical barrier between ileum and colon and protects proximal reflux of colonic contents and bacteria46,47. Diseases like Crohn’s disease(CD) and tuberculosis might effect IC valve and impair its natural barrier function, thereby promoting retrograde migration of bacteria. A study from North India found SIBO to be more common in patients with CD (18.6% vs 1.5%) but not in ulcerative colitis (4.4%) compared to healthy controls48. Obesity surgeries like roux-en-Y gastric bypass increase the risk of SIBO by affecting protective gastric factors (acid content) along with altered motility. Prevalence of SIBO in these patients was found to be around 40%49,50. However, obesity itself has been associated with higher risk of SIBO51.
v) Immune dysfunction: GI tract is protected from bacterial pathogens by integrity of mucosal surface and innate and adaptive immune system. Disruption of gut barrier function and impaired immune response (e.g., IgA secretion) may predispose to SIBO.Patients with disorders like IgA deficiency, common variable immune deficiency, T cell defects, acquired immune deficiency syndrome, etc. are prone to develop SIBO52.
vi) Other conditions: The risk of SIBO has been noted to be high in several other conditions (Table 1). The underlying reason may be a combination of factors such as dysmotility, impaired gastrointestinal secretion, abnormal gut permeability, etc. Growth stunting and intestinal inflammation (environmental enteropathy) have been noted to be frequent in children in low and middle income countries and SIBO has been proposed as one of the contributing factor13,53. In a study from Bangladesh, SIBO was noted in 16.7% children and was associated with elevated intestinal inflammatory marker54. There is increased risk of SIBO in celiac disease. In a meta-analysis, the pooled prevalence of SIBO in celiac disease was 20% (95% CI, 10-30) and was higher in patients with persistent symptoms after gluten free diet compared to those without symptoms (28% vs 10%)55. Gut microflora has been noted to be altered in NASH and the risk of SIBO is increased. A meta-analysis showed the pooled prevalence of SIBO to be 0.41(95% CI, 0.22-0.63) in patient with NASH56.
Clinical Features and Differential Diagnosis
Patients with SIBO can have a wide spectrum of clinical manifestations, from being asymptomatic to having a variable number and severity of symptoms. Some of these symptoms may be related to the underlying risk factor/disease and it may be difficult to attribute the symptoms to SIBO with certainty. A thorough history including the presence of risk factors for SIBO and good clinical examination is essential. The severity of symptoms, features of underlying disease and nutritional status should be assessed. Bloating is the most frequent symptom noted and other common symptoms include diarrhoea, excessive flatulence, gas and abdominal cramps9. Patients with milder disease may manifest with symptoms such as bloating, flatulence and diarrhoea without any features of nutritional deficiency. Less frequently, those with more severe manifestations of SIBO may have hypoalbuminemia, anemia, weight loss, steatorrhea, protein losing enteropathy and deficiencies of various vitamins and minerals3. Carbohydrate fermentation by gut bacteria triggers excessive gas production, leading to symptoms such as bloating and flatulence6. Deconjugation of bile acids leads to malabsorption of fats and fat-soluble vitamins (Vitamin A,D and E)6,15,57. The bacterial products and toxins may injure the mucosal lining of the small intestine causing loss of essential brush border enzymes for nutrient digestion and leakage of proteins6. Bacterial competition for nutrients may lead to deficiencies of vitamin B12, thiamine and niacin. Additionally, bacterial metabolism generates a spectrum of compounds, including vitamin K, folate, D-lactic acid, alcohol, and acetaldehyde6. Consequently, patients with SIBO may have deficiency of vitamin B12 and elevated levels of folate and vitamin K. Patients with SIBO after gastric bypass surgery for obesity also frequently report nausea and vomiting along with bloating and diarrhoea58. Patients with overgrowth of methane producing bacteria may present with constipation13,59. SIBO also affects the course of underlying disease – for example, it is associated with higher relapse rate in patients with IBD13,60. The differential diagnoses for SIBO are varied and depend on the clinical profile of patient and severity of symptoms. In patients with mild disease and non-specific symptoms like bloating, abdominal distension, altered bowel habits, etc. functional gastrointestinal disorders like IBS and functional dyspepsia may be considered11,61. In patients with more severe manifestations like weight loss and malabsorption, differentials include celiac disease, IBD, intestinal tuberculosis, parasitic infestations, tropical sprue, intestinal lymphomas, common variable immunodeficiency, etc.62.
Diagnosis of SIBO
Diagnosis of SIBO requires demonstration of an increase in the number of bacteria or increase in their metabolic byproduct63. Elevated serum folate levels and low vitamin B12 levels, although non-specific, may support a diagnosis of SIBO11,63.
Quantitative culture of small bowel aspirate: Quantitative small bowel aspirate culture is considered the gold standard test for diagnosing SIBO11. This entails collection of duodenal or jejunal content during endoscopy by using a sterile catheter which are then cultured by a serial dilution technique12. A custom double lumen protected catheter which prevents the risk of oropharyngeal contamination while passing the catheter through the scope may be preferred to obtain samples63,64. The small bowel bacterial load in healthy individuals, is generally < 102 CFU/ml65. As the duodenal lumen is more acidic than jejunum, the bacterial load is even lower than that of the jejunum. A recent North American consensus recommended a colony count of greater than 103 CFU/ml for diagnosing SIBO10. Culture techniques can only identify up to 40% of bacterial population as many bacterial species are difficult to grow in culture and there is need for culture independent techniques. Molecular techniques to identify bacterial genomic sequences may overcome this limitation and provide alternative method to diagnose SIBO and also provide added information on the nature/function of bacterial population and even antimicrobial susceptibility. A recent study utilising this technique identified a 4-fold higher abundance of Proteobacteria and a 1.6-fold lower abundance of Firmicutes in SIBO patients compared to those without SIBO64. Another report found a reduction in bacterial alpha diversity and increase in abundance of Streptococcus and Bacteroidetes in duodenal content in SIBO patients66. An increase in metabolic pathways for carbohydrate fermentation, hydrogen and hydrogen sulfide production has also been noted on genomic sequencing studies in this condition67. Interestingly, studies comparing genomic sequencing with cultures of duodenal aspirates support a cutoff of > 103CFU/ml as appropriate to diagnose SIBO1,68. While next generation sequencing (NGS) is a promising method, quantitative estimation of bacterial population with sequencing is expensive and requires special laboratory facilities which are currently not available freely38,69. Although quantitative culture is the gold standard test, its invasive nature, inability to grow all bacterial species, higher cost and risk of oropharyngeal contamination during sample collections are important limitations.
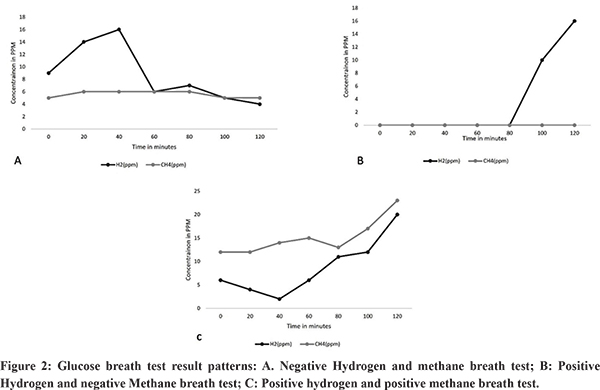
Breath tests: Breath tests are frequently used to diagnose SIBO due to their non-invasive nature 70,71. These tests are based on the production of hydrogen, methane and/or hydrogen sulfide gas by the gut flora (bacteria and archaea) from substrates like glucose and lactulose72. Human cells do not produce hydrogen or methane from carbohydrates. Breath hydrogen and methane levels are recorded every 10 to 15 minutes for 2-4 hours, after ingestion of either 75-100 g of glucose in 20 ml of water (Glucose hydrogen breath test – GHBT) or 10 g of lactulose in a 15 ml solution (Lactulose hydrogen breath test – LHBT)7,71. Up to 30% of general population may not release enough hydrogen in breath due to its utilization by bacteria to produce methane73. Hence simultaneous methane assessment improves the sensitivity of the test. Prior to the breath tests, the patient should not be on antibiotic, probiotic, motility affecting drugs and laxatives. They should avoid smoking, taking complex carbohydrate and dairy products before the test3,70,74. Glucose may be less accurate in detecting distal small bowel bacterial overgrowth as it may be absorbed in the proximal bowel while lactulose may affect gut motility and can influence the result of the test3. In diabetics, glucose may cause hyperglycemia and lactulose may be preferred as a substrate for the test75. Conventionally, a double peak in breath hydrogen after lactulose administration has been described in SIBO. The first peak is due to the production of hydrogen by the excessive small intestinal bacteria and the second by the resident colonic flora and an abnormal first peak is noted in SIBO. There are variations in cut-offs which have been used to diagnose SIBO. The North American consensus defines a positive breath test when there is a>20 part per million (PPM) increase of hydrogen from baseline and a > 10 PPM increase of methane from baseline, within 90 minutes after administration of lactulose (Figure 2)10. The interval after which the level of gas rises depends on the orocaecal transit time which may vary among individuals. A Technetium scintigraphy along with LHBT can be used to identify the entry of the substrate ingested into the caecum to improve the accuracy of this test.
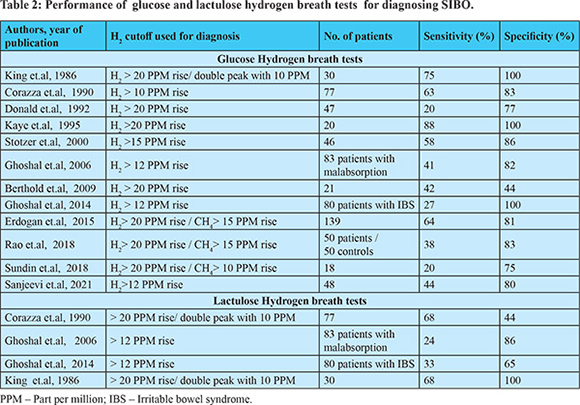
The overall sensitivity of breath tests to diagnose SIBO is not very good and varies based on the substrate and the cut-offs used36,76-87 (Table 2). A recent systematic review and meta-analysis of breath tests for the diagnosis of SIBO showed a pooled sensitivity and specificity of 54.5% and 83.2% and 42% and 70.6% respectively for GHBT and LHBT76. The sensitivity increased to 61.7% and specificity increased to 86% when a cut off of > 12 PPM rise in H2 was used to diagnose SIBO. Accordingly the Asian Pacific consensus advocates a cut off of > 12 PPM rise in H2 after glucose administration for the diagnosis of SIBO11. Breath tests are simple, non-invasive, less expensive and the results are available quickly. More recently capsule based devices have become available which have sensors for directly measuring the level of gases (hydrogen, methane, carbon dioxide, oxygen) in the small bowel88. On comparing results with breath tests, a 3000 times higher concentration of hydrogen was measured by the capsule device. Additionally, the transit times can be estimated based on changes in the concentration of gases. As the performance of breath test is not very accurate, more than one test may be used if clinical suspicion is high75. There are also suggestions for empirical antibiotic therapy if pre-test probability for diagnosis of SIBO is high89.
Detection of radioactive isotope of carbons in breath after ingestion of 14C-D-xylose or 14C-bile acid have also been described to diagnose SIBO90. However, they are rarely used in clinical practice due to the requirement of radioactive material and lower sensitivity compared to culture91. Hydrogen sulfide is also produced by bacteria in gut and a level of =3 ppm at any point during the test is considered positive for diagnosing SIBO. There is limited data on the performance of this test for SIBO9. In addition to detecting bacterial products in breath, presence of metabolites in urine (e.g., bacterially metabolized synthetic bile acid conjugates) have also been evaluated for diagnosing SIBO although more data is required90. Endoscopy and mucosal biopsy are generally not useful for diagnosing SIBO. The small intestinal mucosal surface is generally normal in appearance but in severe cases erythema/friability of mucosa may be present. Histopathology is also normal (on non-specific) in majority of cases although villous blunting/atrophy may be present in some11. The inflammatory markers are also within normal limits63. Apart from diagnosing SIBO, investigations should be done to detect the underlying cause and risk factor for SIBO.
Management of SIBO
Treatment of SIBO is aimed at correcting the overgrowth of bacterial population in small bowel9,11,63. In addition, the factor predisposing to SIBO should be corrected if feasible and the nutritional deficiencies should be replenished9,11,63. Other coexisting conditions contributing to symptoms such as IBS, celiac disease, food intolerance, etc.also need to be recognized and managed.
Antimicrobial therapy: The microorganisms responsible for SIBO include both anaerobic and aerobic organisms and gram positive and negative organisms. Many of them are difficult to grow in culture and hence culture/sensitivity guided therapy is not feasible8. Therefore, broad spectrum antimicrobials are used empirically for therapy. Antibiotics are the mainstay of therapy for SIBO. These include – rifaximin, metronidazole, ciprofloxacin, norfloxacin, doxycycline, tetracycline, neomycin, amoxicillin/clavulanate, trimethoprim-sulfamethoxazole, etc. (Figure 3)8. Antibiotics are given for 7 to 10 days and lead to improvement of symptoms in 46-90% cases and normalization of HBT in 20-75%63.
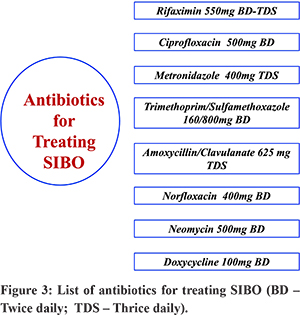
Currently, maximum evidence of efficacy is available for rifaximin and it is generally the preferred antibiotic for treatment of SIBO8,92-94. Rifaximin inhibits bacterial RNA(ribonucleic acid) synthesis and is effective against a broad spectrum of organisms. Only minimal amount (<0.1%) is absorbed from gut and hence it has negligible systemic effects95. Most of the other antibiotics are absorbed in GI tract (systemic) and are less frequently used. In a prospective randomized study on 142 patients with SIBO, rifaximin led to a higher rate of normal GHBT compared to metronidazole (63.4% vs 43.7%, p <0.05) and had lower rate of adverse effects96. A meta-analysis (24 cohort studies, 6 RCT, 1 randomized crossover study, total 1331 patients) on rifaximin showed the pooled SIBO eradication rate of 70.8% (61.4-78.2) based on hydrogen breath test97. Symptoms improved in 67.7% (44.7-86.9) patients in whom HBT became normal. The rate of adverse effect was 4.6% (2.3-7.5). The data from ten studies on patients with IBS in this meta-analysis showed an eradication rate of 71.6% (56.7-84.4). In patients with IBS-D, presence of SIBO confirmed by breath test predicted response of symptoms to antibiotic98,99. Although rifaximin is the preferred antibiotic, there is some evidence favoring metronidazole over rifaximin in patients with blind loop syndrome8,100. In a report from India, sensitivity to quinolones were reported to be better than to tetracycline, ampicillin, cotrimoxazole although rifaximin was not included in this study12. Many of the studies evaluating antibiotics in SIBO have small sample size, lack placebo arm and are non-randomized and hence the quality of evidence is not very strong93. SIBO may be challenging to manage in patients with systemic sclerosis as the underlying disease is difficult to treat although antibiotics are effective in some patients101. The data in pediatric population with SIBO is limited but antibiotics (rifaximin, metronidazole) are effective in about two-thirds of them102. A single course of antibiotic is generally recommended with repeat therapy reserved for those with recurrence of symptoms. A recently published large retrospective study (n=223) from France found rotating antibiotic to be more effective than a single course of antibiotic (70% vs 50.8%, p=0.05)103. In the rotating antibiotic group, metronidazole was given for 10 days of a month alternating with quinolone for 10 days a month for 3 months. The single antibiotic group received any one of these antibiotics for 10 days only. Rifaximin was not used in this study and the success rate of 70% with rotating antibiotic is similar to the success rate with single course of rifaximin97,103. The success of therapy may be determined based on clinical parameters (improvement of bloating, abdominal pain, diarrhoea) and normalization of HBT result. However, in clinical practice, a repeat HBT may not be always required if symptoms have resolved. More recently efforts are being made to develop patient-reported outcome measures in SIBO104.
Probiotics: Apart from antibiotics, probiotics have also been used, both as a standalone treatment or as add on therapy to antibiotic. A systematic review and meta-analysis evaluated the role of probiotic in preventing and treating SIBO (diagnosed by HBT)105. There was no significant benefit of probiotic compared to no probiotic in reducing the incidence of SIBO (four studies). For treating SIBO, when probiotic was used alone the eradication rate was only 53.2% (40.1-65.9). Probiotics were more effective for pain relief but had a lesser impact on the frequency of stool. When probiotics were added to antibiotics (two studies) the eradication rate was 85.8%. The studies on probiotics have limitations in methodology and current guidelines do not recommend probiotics as a standard therapy for SIBO9,11,63. In addition, there are reports of risk of D lactic acidosis with probiotics74,106,107.
Dietary therapy: Growth of bacteria requires appropriate nutrients, and one of the therapeutic options may be to limit the amount of fermentable nutrients available to the bacteria by dietary modification. In a study by Pimental et al., elemental diet led to the normalization of LBT in 85% of the 93 patients with SIBO by day 21. Normalization of LBT was associated with higher rate of symptom improvement108. The benefit of elemental diet may be due to its predominant absorption in the proximal small bowel which leaves less nutrients for bacteria to grow108. Elemental diets are less palatable and are expensive and more evidence is required on their efficacy before making firm recommendations. Diet with low FODMAP (Fermentable oligosaccharides, disaccharides, monosaccharides and polyols) content have been shown to be effective in patients with IBS but adequate data on managing SIBO with this diet is awaited11,95. In addition to specific interventions, general dietary measures may be helpful in some patients. For example, reducing intake of carbonated beverages and non-absorbable sugars may help reduce symptoms of bloating and flatulence9,95. Avoiding milk may minimize symptoms of lactose maldigestion (bloating, flatulence, diarrhea) in patients with lactase deficiency7. Vegan diet may also have a beneficial effect on microbial composition109.
Other therapies: A recent randomized placebo controlled study evaluated fecal microbiota transplantation (FMT, one capsule weekly for 4 weeks) for treating SIBO in 55 patients110. FMT was better than placebo in alleviating gastrointestinal symptoms. However more data is required. A small study found a three week course of octreotide to be effective in treating SIBO in systemic sclerosis111. Alternative treatments such as herbal therapies have also been tried in SIBO106. A metanalysis on alternative treatments for SIBO found limited benefit of herbal therapy in SIBO112.
Recurrence of symptoms: Following initial successful therapy, about half of the patients have recurrence of symptoms within one year and hence they require close follow up and repeat treatment if required8,113. Risk of recurrence is high in patients with persistent underlying cause, old age, those using long term PPI and in patients with history of appendectomy8. For recurrent symptoms, rifaximin may be used again if this was efficacious earlier11,114. If symptoms recur frequently(>4 episodes per year), cyclical antibiotic has been suggested rather than the same antibiotic every month to reduce the risk of antimicrobial resistance38,63. Cyclical antibiotics may be given for 7 to 10 days per month and can include two or three antibiotics (metronidazole, rifaximin, quinolone, etc.) with one of them being given each month63.
Management of nutritional deficiencies: Symptoms of SIBO often appear before nutritional deficiencies are apparent but they must be carefully looked for3,9. The nutritional deficiencies should be identified by both clinical assessment and laboratory evaluation for both macronutrients and micronutrients3. There may be deficiency of vitamin B12, iron and fat soluble vitamins3. Deficient nutrients should be appropriately replaced.
Correction of predisposing factors: This is an important component of management of SIBO. Patients with anatomical abnormalities (e.g., blind loop, fistula, diverticula or obstruction) may need surgical intervention. Improving the motility of the intestine with prokinetics (e.g., domperidone, erythromycin, itopride, etc.) has been suggested in patients with dysmotility although there is limited evidence for their efficacy8,74,115. Another important consideration is to avoid medication associated with risk of SIBO (e.g., PPI, opioid, etc.).
Identification of coexisting conditions causing symptoms: About one-third of patients with symptoms suggestive of SIBO may not respond to treatment74. Apart from failure of antibiotics, the cause of symptoms may be a coexisting condition like IBS, celiac disease, bile salt malabsorption, food(e.g. lactose) intolerance, drugs, etc. and this must be identified and managed accordingly74.
Intestinal methanogen overgrowth (IMO)
Methane producing organisms are present in up to two thirds of individuals. These organisms belong to the domain archaea and are distinct from bacteria and hence the term intestinal methanogen overgrowth (IMO) has been proposed9. In IMO, there is overproduction of methane, mostly by the archaea Methanobrevibacter smithii9,116. Methane inhibits intestinal motility and has been associated with chronic constipation. In a meta-analysis, methane positive SIBO was more frequent in IBS-C compared to IBS-D (OR 3.1, 1.7-5.6)59. Treatment with antibiotics has shown benefit in improving bowel movement59,117,118. In a study from India on patients with chronic constipation, rifaximin improved symptoms of constipation by reducing methane production and improving colonic transit time117. Combination of rifaximin and neomycin may have better efficacy than rifaximin alone118,119. Statins have also been found to reduce methane production by Methanobrevibacter but more data is required118,120.
Conclusion
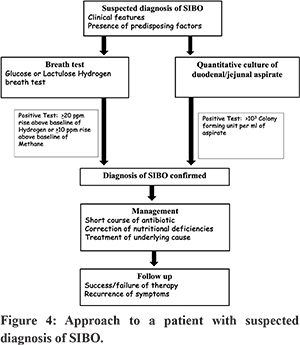
SIBO occurs in a variety of clinical settings and due to the non-specific nature of the symptoms, a high index of clinical suspicion is required for diagnosis. Figure 4 outlines the approach to a patient with suspected SIBO. Diagnosis can be made by quantitative culture of small bowel aspirate or breath tests. Treatment with short course of antibiotics is often effective in reducing overgrowth of bacteria but treatment of underlying cause is needed to prevent recurrence.
References - Leite G, Morales W, Weitsman S, Celly S, Parodi G, Mathur R, et al. The duodenal microbiome is altered in small intestinal bacterial overgrowth. PLoS One. 2020;15(7):e0234906.
- Nagasue T, Hirano A, Torisu T, Umeno J, Shibata H, Moriyama T, et al. The Compositional Structure of the Small Intestinal Microbial Community via Balloon-Assisted Enteroscopy. Digestion. 2022;103(4):308-18.
- Adike A, DiBaise JK. Small Intestinal Bacterial Overgrowth: Nutritional Implications, Diagnosis, and Management. Gastroenterol Clin North Am. 2018;47(1):193-208.
- Simren M, Barbara G, Flint HJ, Spiegel BM, Spiller RC, Vanner S, et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62(1):159-76.
- Bouhnik Y, Alain S, Attar A, Flourie B, Raskine L, Sanson-Le Pors MJ, et al. Bacterial populations contaminating the upper gut in patients with small intestinal bacterial overgrowth syndrome. Am J Gastroenterol. 1999;94(5):1327-31.
- Bushyhead D, Quigley EMM. Small Intestinal Bacterial Overgrowth-Pathophysiology and Its Implications for Definition and Management. Gastroenterology. 2022;163(3):593-607.
- Ghoshal UC, Ghoshal U. Small Intestinal Bacterial Overgrowth and Other Intestinal Disorders. Gastroenterol Clin North Am. 2017;46(1):103-20.
- Bures J, Cyrany J, Kohoutova D, Forstl M, Rejchrt S, Kvetina J, et al. Small intestinal bacterial overgrowth syndrome. World J Gastroenterol. 2010;16(24):2978-90.
- Pimentel M, Saad RJ, Long MD, Rao SSC. ACG Clinical Guideline: Small Intestinal Bacterial Overgrowth. Am J Gastroenterol. 2020;115(2):165-78.
- Rezaie A, Buresi M, Lembo A, Lin H, McCallum R, Rao S, et al. Hydrogen and Methane-Based Breath Testing in Gastrointestinal Disorders: The North American Consensus. Am J Gastroenterol. 2017;112(5):775-84.
- Ghoshal UC, Sachdeva S, Ghoshal U, Misra A, Puri AS, Pratap N, et al. Asian-Pacific consensus on small intestinal bacterial overgrowth in gastrointestinal disorders: An initiative of the Indian Neurogastroenterology and Motility Association. Indian J Gastroenterol. 2022;41(5):483-507.
- Ghoshal U, Ghoshal UC, Ranjan P, Naik SR, Ayyagari A. Spectrum and antibiotic sensitivity of bacteria contaminating the upper gut in patients with malabsorption syndrome from the tropics. BMC Gastroenterol. 2003;3:9.
- Efremova I, Maslennikov R, Poluektova E, Vasilieva E, Zharikov Y, Suslov A, et al. Epidemiology of small intestinal bacterial overgrowth. World J Gastroenterol. 2023;29(22):3400-21.
- Grace E, Shaw C, Whelan K, Andreyev HJ. Review article: small intestinal bacterial overgrowth--prevalence, clinical features, current and developing diagnostic tests, and treatment. Aliment Pharmacol Ther. 2013;38(7):674-88.
- Dukowicz AC, Lacy BE, Levine GM. Small intestinal bacterial overgrowth: a comprehensive review. Gastroenterol Hepatol (N Y). 2007;3(2):112-22.
- Saffouri GB, Shields-Cutler RR, Chen J, Yang Y, Lekatz HR, Hale VL, et al. Small intestinal microbial dysbiosis underlies symptoms associated with functional gastrointestinal disorders. Nat Commun. 2019;10(1):2012.
- Chen B, Kim JJ, Zhang Y, Du L, Dai N. Prevalence and predictors of small intestinal bacterial overgrowth in irritable bowel syndrome: a systematic review and meta-analysis. J Gastroenterol. 2018;53(7):807-18.
- Sachdeva S, Rawat AK, Reddy RS, Puri AS. Small intestinal bacterial overgrowth (SIBO) in irritable bowel syndrome: frequency and predictors. J Gastroenterol Hepatol. 2011;26 Suppl 3:135-8.
- Ghoshal UC, Kumar S, Mehrotra M, Lakshmi C, Misra A. Frequency of small intestinal bacterial overgrowth in patients with irritable bowel syndrome and chronic non-specific diarrhea. J Neurogastroenterol Motil. 2010;16(1):40-6.
- Banik GD, Maity A, Som S, Ghosh C, Daschakraborty SB, Chaudhuri S, et al. Diagnosis of small intestinal bacterial overgrowth in irritable bowel syndrome patients using high-precision stable13CO2/12CO2isotope ratios in exhaled breath. J Anal At Spectrom. 2014;29(10):1918-24.
- Rana SV, Sinha SK, Sikander A, Bhasin DK, Singh K. Study of small intestinal bacterial overgrowth in North Indian patients with irritable bowel syndrome: a case control study. Trop Gastroenterol. 2008;29(1):23-5.
- Gurusamy SR, Shah A, Talley NJ, Koloski N, Jones MP, Walker MM, et al. Small Intestinal Bacterial Overgrowth in Functional Dyspepsia: A Systematic Review and Meta-Analysis. Am J Gastroenterol. 2021;116(5):935-42.
- Roland BC, Ciarleglio MM, Clarke JO, Semler JR, Tomakin E, Mullin GE, et al. Small Intestinal Transit Time Is Delayed in Small Intestinal Bacterial Overgrowth. J Clin Gastroenterol. 2015;49(7):571-6.
- Kruszewska D, Ljungh A, Hynes SO, Pierzynowski SG. Effect of the antibacterial activity of pig pancreatic juice on human multiresistant bacteria. Pancreas. 2004;28(2):191-9.
- Binder HJ, Filburn B, Floch M. Bile acid inhibition of intestinal anaerobic organisms. Am J Clin Nutr. 1975;28(2):119-25.
- Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M, et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci U S A. 2006;103(10):3920-5.
- Su T, Lai S, Lee A, He X, Chen S. Meta-analysis: proton pump inhibitors moderately increase the risk of small intestinal bacterial overgrowth. J Gastroenterol. 2018;53(1):27-36.
- Dutta AK, Jain A, Jearth V, Mahajan R, Panigrahi MK, Sharma V, et al. Guidelines on optimizing the use of proton pump inhibitors: PPI stewardship. Indian J Gastroenterol. 2023;42(5):601-28.
- Kakiyama G, Pandak WM, Gillevet PM, Hylemon PB, Heuman DM, Daita K, et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol. 2013;58(5):949-55.
- Ghosh G, Jesudian AB. Small Intestinal Bacterial Overgrowth in Patients With Cirrhosis. J Clin Exp Hepatol. 2019;9(2):257-67.
- Pande C, Kumar A, Sarin SK. Small-intestinal bacterial overgrowth in cirrhosis is related to the severity of liver disease. Aliment Pharmacol Ther. 2009;29(12):1273-81.
- Maslennikov R, Pavlov C, Ivashkin V. Small intestinal bacterial overgrowth in cirrhosis: systematic review and meta-analysis. Hepatol Int. 2018;12(6):567-76.
- Lee AA, Baker JR, Wamsteker EJ, Saad R, DiMagno MJ. Small Intestinal Bacterial Overgrowth Is Common in Chronic Pancreatitis and Associates With Diabetes, Chronic Pancreatitis Severity, Low Zinc Levels, and Opiate Use. Am J Gastroenterol. 2019;114(7):1163-71.
- Ni Chonchubhair HM, Bashir Y, Dobson M, Ryan BM, Duggan SN, Conlon KC. The prevalence of small intestinal bacterial overgrowth in non-surgical patients with chronic pancreatitis and pancreatic exocrine insufficiency (PEI). Pancreatology. 2018;18(4):379-85.
- Therrien A, Bouchard S, Sidani S, Bouin M. Prevalence of Small Intestinal Bacterial Overgrowth among Chronic Pancreatitis Patients: A Case-Control Study. Can J Gastroenterol Hepatol. 2016;2016:7424831.
- Sanjeevi R, Jamwal KD, Dhar Chowdhury S, Ramadass B, Gayathri R, Dutta AK, et al. Assessment of small intestinal bacterial overgrowth in chronic pancreatitis patients using jejunal aspirate culture and glucose hydrogen breath test. Scand J Gastroenterol. 2021;56(5):588-93.
- El Kurdi B, Babar S, El Iskandarani M, Bataineh A, Lerch MM, Young M, et al. Factors That Affect Prevalence of Small Intestinal Bacterial Overgrowth in Chronic Pancreatitis: A Systematic Review, Meta-Analysis, and Meta-Regression. Clin Transl Gastroenterol. 2019;10(9):e00072.
- Ahmed JF, Padam P, Ruban A. Aetiology, diagnosis and management of small intestinal bacterial overgrowth. Frontline Gastroenterol. 2023;14(2):149-54.
- Capurso G, Signoretti M, Archibugi L, Stigliano S, Delle Fave G. Systematic review and meta-analysis: Small intestinal bacterial overgrowth in chronic pancreatitis. United European Gastroenterol J. 2016;4(5):697-705.
- Pimentel M, Soffer EE, Chow EJ, Kong Y, Lin HC. Lower frequency of MMC is found in IBS subjects with abnormal lactulose breath test, suggesting bacterial overgrowth. Dig Dis Sci. 2002;47(12):2639-43.
- Choung RS, Ruff KC, Malhotra A, Herrick L, Locke GR, 3rd, Harmsen WS, et al. Clinical predictors of small intestinal bacterial overgrowth by duodenal aspirate culture. Aliment Pharmacol Ther. 2011;33(9):1059-67.
- Malik A, Morya RK, Bhadada SK, Rana S. Type 1 diabetes mellitus: Complex interplay of oxidative stress, cytokines, gastrointestinal motility and small intestinal bacterial overgrowth. Eur J Clin Invest. 2018;48(11):e13021.
- Feng X, Li XQ. The prevalence of small intestinal bacterial overgrowth in diabetes mellitus: a systematic review and meta-analysis. Aging (Albany NY). 2022;14(2):975-88.
- Shah A, Pakeerathan V, Jones MP, Kashyap PC, Virgo K, Fairlie T, et al. Small Intestinal Bacterial Overgrowth Complicating Gastrointestinal Manifestations of Systemic Sclerosis: A Systematic Review and Meta-analysis. J Neurogastroenterol Motil. 2023;29(2):132-44.
- Beas R, Riva-Moscoso A, Montalvan-Sanchez E, Principe-Meneses FS, Aljaras R, Ramirez-Rojas M, et al. Prevalence of small intestinal bacterial overgrowth in patients with gastroparesis: a systematic review and meta-analysis. Gastroenterol Hepatol Bed Bench. 2023;16(1):438-47.
- Miller LS, Vegesna AK, Sampath AM, Prabhu S, Kotapati SK, Makipour K. Ileocecal valve dysfunction in small intestinal bacterial overgrowth: a pilot study. World J Gastroenterol. 2012;18(46):6801-8.
- Roland BC, Ciarleglio MM, Clarke JO, Semler JR, Tomakin E, Mullin GE, et al. Low ileocecal valve pressure is significantly associated with small intestinal bacterial overgrowth (SIBO). Dig Dis Sci. 2014;59(6):1269-77.
- Ghoshal UC, Yadav A, Fatima B, Agrahari AP, Misra A. Small intestinal bacterial overgrowth in patients with inflammatory bowel disease: A case-control study. Indian J Gastroenterol. 2022;41(1):96-103.
- Sabate JM, Coupaye M, Ledoux S, Castel B, Msika S, Coffin B, et al. Consequences of Small Intestinal Bacterial Overgrowth in Obese Patients Before and After Bariatric Surgery. Obes Surg. 2017;27(3):599-605.
- Novljan U, Pintar T. Small Intestinal Bacterial Overgrowth in Patients with Roux-en-Y Gastric Bypass and One-Anastomosis Gastric Bypass. Obes Surg. 2022;32(12):4102-9.
- Wijarnpreecha K, Werlang ME, Watthanasuntorn K, Panjawatanan P, Cheungpasitporn W, Gomez V, et al. Obesity and Risk of Small Intestine Bacterial Overgrowth: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2020;65(5):1414-22.
- Ghoshal UC, Goel A, Ghoshal U, Jain M, Misra A, Choudhuri G. Chronic diarrhea and malabsorption due to hypogammaglobulinemia: a report on twelve patients. Indian J Gastroenterol. 2011;30(4):170-4.
- Collard JM, Andrianonimiadana L, Habib A, Rakotondrainipiana M, Andriantsalama P, Randriamparany R, et al. High prevalence of small intestine bacteria overgrowth and asymptomatic carriage of enteric pathogens in stunted children in Antananarivo, Madagascar. PLoS Negl Trop Dis. 2022;16(5):e0009849.
- Donowitz JR, Haque R, Kirkpatrick BD, Alam M, Lu M, Kabir M, et al. Small Intestine Bacterial Overgrowth and Environmental Enteropathy in Bangladeshi Children. mBio. 2016;7(1):e02102-15.
- Losurdo G, Marra A, Shahini E, Girardi B, Giorgio F, Amoruso A, et al. Small intestinal bacterial overgrowth and celiac disease: A systematic review with pooled-data analysis. Neurogastroenterol Motil. 2017;29(6).
- Gudan A, Jamiol-Milc D, Hawrylkowicz V, Skonieczna-Zydecka K, Stachowska E. The Prevalence of Small Intestinal Bacterial Overgrowth in Patients with Non-Alcoholic Liver Diseases: NAFLD, NASH, Fibrosis, Cirrhosis-A Systematic Review, Meta-Analysis and Meta-Regression. Nutrients. 2022;14(24).
- Bala L, Ghoshal UC, Ghoshal U, Tripathi P, Misra A, Gowda GA, et al. Malabsorption syndrome with and without small intestinal bacterial overgrowth: a study on upper-gut aspirate using 1H NMR spectroscopy. Magn Reson Med. 2006;56(4):738-44.
- Dolan RD, Baker J, Harer K, Lee A, Hasler W, Saad R, et al. Small Intestinal Bacterial Overgrowth: Clinical Presentation in Patients with Roux-en-Y Gastric Bypass. Obes Surg. 2021;31(2):564-9.
- Gandhi A, Shah A, Jones MP, Koloski N, Talley NJ, Morrison M, et al. Methane positive small intestinal bacterial overgrowth in inflammatory bowel disease and irritable bowel syndrome: A systematic review and meta-analysis. Gut Microbes. 2021;13(1):1933313.
- Efremova I, Maslennikov R, Alieva A, Poluektova E, Ivashkin V. Small Intestinal Bacterial Overgrowth Is Associated with Poor Prognosis in Cirrhosis. Microorganisms. 2023;11(4).
- Jacobs C, Coss Adame E, Attaluri A, Valestin J, Rao SS. Dysmotility and proton pump inhibitor use are independent risk factors for small intestinal bacterial and/or fungal overgrowth. Aliment Pharmacol Ther. 2013;37(11):1103-11.
- Ramakrishna BS, Venkataraman S, Mukhopadhya A. Tropical malabsorption. Postgrad Med J. 2006;82(974):779-87.
- Quigley EMM, Murray JA, Pimentel M. AGA Clinical Practice Update on Small Intestinal Bacterial Overgrowth: Expert Review. Gastroenterology. 2020;159(4):1526-32.
- Leite GGS, Morales W, Weitsman S, Celly S, Parodi G, Mathur R, et al. Optimizing microbiome sequencing for small intestinal aspirates: validation of novel techniques through the REIMAGINE study. BMC Microbiol. 2019;19(1):239.
- Khoshini R, Dai SC, Lezcano S, Pimentel M. A systematic review of diagnostic tests for small intestinal bacterial overgrowth. Dig Dis Sci. 2008;53(6):1443-54.
- Bamba S, Imai T, Sasaki M, Ohno M, Yoshida S, Nishida A, et al. Altered gut microbiota in patients with small intestinal bacterial overgrowth. J Gastroenterol Hepatol. 2023;38(1):61-9.
- Leite G, Rezaie A, Mathur R, Barlow GM, Rashid M, Hosseini A, et al. Defining Small Intestinal Bacterial Overgrowth by Culture and High Throughput Sequencing. Clin Gastroenterol Hepatol. 2023.
- Leite G, Villanueva-Millan MJ, Celly S, Sedighi R, Morales W, Rezaie A, et al. 4 – First Large Scale Study Defining the Characteristic Microbiome Signatures of Small Intestinal Bacterial Overgrowth (SIBO): Detailed Analysis from the Reimagine Study. Gastroenterology. 2019;156(6):S-1-S-2.
- Giamarellos-Bourboulis E, Tang J, Pyleris E, Pistiki A, Barbatzas C, Brown J, et al. Molecular assessment of differences in the duodenal microbiome in subjects with irritable bowel syndrome. Scand J Gastroenterol. 2015;50(9):1076-87.
- Gasbarrini A, Corazza GR, Gasbarrini G, Montalto M, Di Stefano M, Basilisco G, et al. Methodology and indications of H2-breath testing in gastrointestinal diseases: the Rome Consensus Conference. Aliment Pharmacol Ther. 2009;29 Suppl 1:1-49.
- Ghoshal UC. How to interpret hydrogen breath tests. J Neurogastroenterol Motil. 2011;17(3):312-7.
- Rana SV, Malik A. Hydrogen breath tests in gastrointestinal diseases. Indian J Clin Biochem. 2014;29(4):398-405.
- Saad RJ, Chey WD. Breath testing for small intestinal bacterial overgrowth: maximizing test accuracy. Clin Gastroenterol Hepatol. 2014;12(12):1964-72; quiz e119-20.
- Rao SSC, Bhagatwala J. Small Intestinal Bacterial Overgrowth: Clinical Features and Therapeutic Management. Clin Transl Gastroenterol. 2019;10(10):e00078.
- Bhagatwala J, Sharma A, Leelasinjaroen P, Tetangco E, De Andino NM, Rao SS. 199 - Investigation of Small Intestinal Bacterial Overgrowth (SIBO) in Diabetics Using Fructose Breath Test. Gastroenterology. 2018;154(6):S-53-S-4.
- Losurdo G, Leandro G, Ierardi E, Perri F, Barone M, Principi M, et al. Breath Tests for the Non-invasive Diagnosis of Small Intestinal Bacterial Overgrowth: A Systematic Review With Meta-analysis. J Neurogastroenterol Motil. 2020;26(1):16-28.
- King CE, Toskes PP. Comparison of the 1-gram [14C]xylose, 10-gram lactulose-H2, and 80-gram glucose-H2 breath tests in patients with small intestine bacterial overgrowth. Gastroenterology. 1986;91(6):1447-51.
- Corazza GR, Menozzi MG, Strocchi A, Rasciti L, Vaira D, Lecchini R, et al. The diagnosis of small bowel bacterial overgrowth. Reliability of jejunal culture and inadequacy of breath hydrogen testing. Gastroenterology. 1990;98(2):302-9.
- Donald IP, Kitchingmam G, Donald F, Kupfer RM. The diagnosis of small bowel bacterial overgrowth in elderly patients. J Am Geriatr Soc. 1992;40(7):692-6.
- Kaye SA, Lim SG, Taylor M, Patel S, Gillespie S, Black CM. Small bowel bacterial overgrowth in systemic sclerosis: detection using direct and indirect methods and treatment outcome. Br J Rheumatol. 1995;34(3):265-9.
- Stotzer PO, Kilander AF. Comparison of the 1-gram (14)C-D-xylose breath test and the 50-gram hydrogen glucose breath test for diagnosis of small intestinal bacterial overgrowth. Digestion. 2000;61(3):165-71.
- Ghoshal UC, Ghoshal U, Das K, Misra A. Utility of hydrogen breath tests in diagnosis of small intestinal bacterial overgrowth in malabsorption syndrome and its relationship with oro-cecal transit time. Indian J Gastroenterol. 2006;25(1):6-10.
- Berthold HK, Schober P, Scheurlen C, Marklein G, Horre R, Gouni-Berthold I, et al. Use of the lactose-[13C]ureide breath test for diagnosis of small bowel bacterial overgrowth: comparison to the glucose hydrogen breath test. J Gastroenterol. 2009;44(9):944-51.
- Ghoshal UC, Srivastava D, Ghoshal U, Misra A. Breath tests in the diagnosis of small intestinal bacterial overgrowth in patients with irritable bowel syndrome in comparison with quantitative upper gut aspirate culture. Eur J Gastroenterol Hepatol. 2014;26(7):753-60.
- Erdogan A, Rao SS, Gulley D, Jacobs C, Lee YY, Badger C. Small intestinal bacterial overgrowth: duodenal aspiration vs glucose breath test. Neurogastroenterol Motil. 2015;27(4):481-9.
- Rao SSC, Tan G, Abdulla H, Yu S, Larion S, Leelasinjaroen P. Does colectomy predispose to small intestinal bacterial (SIBO) and fungal overgrowth (SIFO)? Clin Transl Gastroenterol. 2018;9(4):146.
- Sundin OH, Mendoza-Ladd A, Morales E, Fagan BM, Zeng M, Diaz-Arevalo D, et al. Does a glucose-based hydrogen and methane breath test detect bacterial overgrowth in the jejunum? Neurogastroenterol Motil. 2018;30(11):e13350.
- Ghoshal UC, Ghoshal U, Shah A, Holtmann G. Evaluation of small intestinal bacterial overgrowth. Expert Rev Gastroenterol Hepatol. 2023;17(5):461-7.
- Arasaradnam RP, Brown S, Forbes A, Fox MR, Hungin P, Kelman L, et al. Guidelines for the investigation of chronic diarrhoea in adults: British Society of Gastroenterology, 3rd edition. Gut. 2018;67(8):1380-99.
- Maeda Y, Murakami T. Diagnosis by Microbial Culture, Breath Tests and Urinary Excretion Tests, and Treatments of Small Intestinal Bacterial Overgrowth. Antibiotics (Basel). 2023;12(2).
- Riordan SM, McIver CJ, Duncombe VM, Bolin TD, Thomas MC. Factors influencing the 1-g 14C-D-xylose breath test for bacterial overgrowth. Am J Gastroenterol. 1995;90(9):1455-60.
- Yang J, Lee HR, Low K, Chatterjee S, Pimentel M. Rifaximin versus other antibiotics in the primary treatment and retreatment of bacterial overgrowth in IBS. Dig Dis Sci. 2008;53(1):169-74.
- Shah SC, Day LW, Somsouk M, Sewell JL. Meta-analysis: antibiotic therapy for small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2013;38(8):925-34.
- Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364(1):22-32.
- Bohm M, Siwiec RM, Wo JM. Diagnosis and management of small intestinal bacterial overgrowth. Nutr Clin Pract. 2013;28(3):289-99.
- Lauritano EC, Gabrielli M, Scarpellini E, Ojetti V, Roccarina D, Villita A, et al. Antibiotic therapy in small intestinal bacterial overgrowth: rifaximin versus metronidazole. Eur Rev Med Pharmacol Sci. 2009;13(2):111-6.
- Gatta L, Scarpignato C. Systematic review with meta-analysis: rifaximin is effective and safe for the treatment of small intestine bacterial overgrowth. Aliment Pharmacol Ther. 2017;45(5):604-16.
- Ghoshal UC, Srivastava D, Misra A, Ghoshal U. A proof-of-concept study showing antibiotics to be more effective in irritable bowel syndrome with than without small-intestinal bacterial overgrowth: a randomized, double-blind, placebo-controlled trial. Eur J Gastroenterol Hepatol. 2016;28(3):281-9.
- Rezaie A, Heimanson Z, McCallum R, Pimentel M. Lactulose Breath Testing as a Predictor of Response to Rifaximin in Patients With Irritable Bowel Syndrome With Diarrhea. Am J Gastroenterol. 2019;114(12):1886-93.
- Di Stefano M, Miceli E, Missanelli A, Mazzocchi S, Corazza GR. Absorbable vs. non-absorbable antibiotics in the treatment of small intestine bacterial overgrowth in patients with blind-loop syndrome. Aliment Pharmacol Ther. 2005;21(8):985-92.
- Pittman N, Rawn SM, Wang M, Masetto A, Beattie KA, Larche M. Treatment of small intestinal bacterial overgrowth in systemic sclerosis: a systematic review. Rheumatology (Oxford). 2018;57(10):1802-11.
- Peinado Fabregat MI, Gardner RM, Hassan MA, Kapphahn K, Yeh AM. Small Intestinal Bacterial Overgrowth in Children: Clinical Features and Treatment Response. JPGN Rep. 2022;3(2):e185.
- Richard N, Desprez C, Wuestenberghs F, Leroi AM, Gourcerol G, Melchior C. The effectiveness of rotating versus single course antibiotics for small intestinal bacterial overgrowth. United European Gastroenterol J. 2021;9(6):645-54.
- Durgam N, Dashputre AA, Moshkovich O, Rezaie A, Martinez N, Enayati P, et al. Content validation of a daily patient-reported outcome measure for assessing symptoms in patients with Small Intestinal Bacterial Overgrowth. Qual Life Res. 2023.
- Zhong C, Qu C, Wang B, Liang S, Zeng B. Probiotics for Preventing and Treating Small Intestinal Bacterial Overgrowth: A Meta-Analysis and Systematic Review of Current Evidence. J Clin Gastroenterol. 2017;51(4):300-11.
- Achufusi TGO, Sharma A, Zamora EA, Manocha D. Small Intestinal Bacterial Overgrowth: Comprehensive Review of Diagnosis, Prevention, and Treatment Methods. Cureus. 2020;12(6):e8860.
- Rao SSC, Rehman A, Yu S, Andino NM. Brain fogginess, gas and bloating: a link between SIBO, probiotics and metabolic acidosis. Clin Transl Gastroenterol. 2018;9(6):162.
- Pimentel M, Constantino T, Kong Y, Bajwa M, Rezaei A, Park S. A 14-day elemental diet is highly effective in normalizing the lactulose breath test. Dig Dis Sci. 2004;49(1):73-7.
- Zimmer J, Lange B, Frick JS, Sauer H, Zimmermann K, Schwiertz A, et al. A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. Eur J Clin Nutr. 2012;66(1):53-60.
- Xu F, Li N, Wang C, Xing H, Chen D, Wei Y. Clinical efficacy of fecal microbiota transplantation for patients with small intestinal bacterial overgrowth: a randomized, placebo-controlled clinic study. BMC Gastroenterol. 2021;21(1):54.
- Soudah HC, Hasler WL, Owyang C. Effect of octreotide on intestinal motility and bacterial overgrowth in scleroderma. N Engl J Med. 1991;325(21):1461-7.
- Nickles MA, Hasan A, Shakhbazova A, Wright S, Chambers CJ, Sivamani RK. Alternative Treatment Approaches to Small Intestinal Bacterial Overgrowth: A Systematic Review. J Altern Complement Med. 2021;27(2):108-19.
- Lauritano EC, Gabrielli M, Scarpellini E, Lupascu A, Novi M, Sottili S, et al. Small intestinal bacterial overgrowth recurrence after antibiotic therapy. Am J Gastroenterol. 2008;103(8):2031-5.
- Lembo A, Pimentel M, Rao SS, Schoenfeld P, Cash B, Weinstock LB, et al. Repeat Treatment With Rifaximin Is Safe and Effective in Patients With Diarrhea-Predominant Irritable Bowel Syndrome. Gastroenterology. 2016;151(6):1113-21.
- Pimentel M, Morales W, Lezcano S, Sun-Chuan D, Low K, Yang J. Low-dose nocturnal tegaserod or erythromycin delays symptom recurrence after treatment of irritable bowel syndrome based on presumed bacterial overgrowth. Gastroenterol Hepatol (N Y). 2009;5(6):435-42.
- Ghoshal U, Shukla R, Srivastava D, Ghoshal UC. Irritable Bowel Syndrome, Particularly the Constipation-Predominant Form, Involves an Increase in Methanobrevibacter smithii, Which Is Associated with Higher Methane Production. Gut Liver. 2016;10(6):932-8.
- Ghoshal UC, Srivastava D, Misra A. A randomized double-blind placebo-controlled trial showing rifaximin to improve constipation by reducing methane production and accelerating colon transit: A pilot study. Indian J Gastroenterol. 2018;37(5):416-23.
- Gottlieb K, Wacher V, Sliman J, Pimentel M. Review article: inhibition of methanogenic archaea by statins as a targeted management strategy for constipation and related disorders. Aliment Pharmacol Ther. 2016;43(2):197-212.
- Low K, Hwang L, Hua J, Zhu A, Morales W, Pimentel M. A combination of rifaximin and neomycin is most effective in treating irritable bowel syndrome patients with methane on lactulose breath test. J Clin Gastroenterol. 2010;44(8):547-50.
- Muskal SM, Sliman J, Kokai-Kun J, Pimentel M, Wacher V, Gottlieb K. Lovastatin lactone may improve irritable bowel syndrome with constipation (IBS-C) by inhibiting enzymes in the archaeal methanogenesis pathway. F1000Res. 2016;5:606.
|