Simmi K Ratan, Kamal Nain Ratan, Kiran Bishnoi1, Atul Jhanwar, Vidhu Kaushik2, Abhishek Makkar
Department of Pediatric Surgery,
Department of Radiodiagnosis1
Department of Pathology2
Pt. B D Sharma PGIMS,
Rohtak, Haryana, India.
Corresponding Author:
Simmi K Ratan
Email: drjohnsimmi@yahoo.com
48uep6bbphidvals|319 48uep6bbph|2000F98CTab_Articles|Fulltext Gall bladder cancer which is common among the females in the northern part of India is an uncommon cancer in most parts of the world. Unfortunately, most of the patients present in advanced and unresectable stage where surgery as definite treatment option may not be offered. Such patients are taken for palliative treatment either by placing a stent to relieve the obstructive jaundice and/ or chemotherapy which do not have a proven role. With chemotherapeutic agents (with or without 5FU) response rates are seen in 0-38% of cases. [1,2,3,4]
Gastric duplication cysts (GDC) are comparatively rare lesions, comprising only 7% of all GIT duplications. They are most commonly encountered along the greater curvature of the stomach and usually present before the age of one year.[1] These lesions need an urgent attention due to a high rate of complications associated with lesions; as bleeding and perforation. However, they can pose diagnostic difficulties (specially with other intra-abdominal cystic lesions) and thus lead to a significant delay in the definitive treatment. We herein describe two children with GDC who were initially misdiagnosed as pancreatic pseudocysts (PPC) due to closely resembling radiological features of both these lesions.
Case reports:
Case no.1
A 10-year-old boy presented with dull epigastric pain following blunt abdominal trauma sustained a week back. He affirmed to have suffered episodes of upper abdominal pain and occasional vomitings for past one year, for which he had received nonspecific treatment from private practitioners. He also had complete rectal prolapse, about 3 cm long, of 6 months duration. Abdominal examination was unremarkable though anal examination revealed a total rectal prolapse with a decreased anal tone. His serum amylase was mildly raised. Ultrasound of the abdomen at our centre showed a cystic mass of about 5x7 cm mainly in relation to the body of the pancreas. The diagnosis of post traumatic pseudocyst of pancreas with rectal prolapse was made at our centre. The prolapse, probably a sequel of constant straining during episodes of abdominal pain, was managed by Thiersch wiring. The patient was discharged with an advice for regular two weekly follow ups for monitoring of the size of pseudocyst.
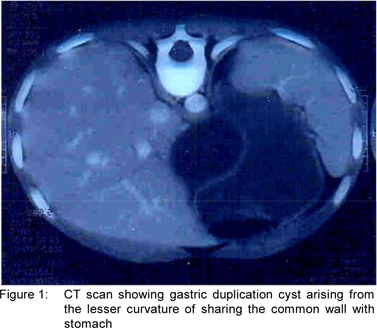
On follow up, the size of the cyst was found to be static and the child continued to be symptomatic. Hence, the child was re-admitted for exploratory laparotomy. At exploration, a large cystic mass measuring10x10 cm in size was found to be arising from the lesser curvature of stomach and was found to be abutting the body of the pancreas. Needle aspiration yielded clear yellowish fluid. Near total excision of cyst was carried out except where it was intimately adherent to the lesser curvature of the stomach; where only mucosal excision was carried out. The histopathology report corroborated with the diagnosis of gastric duplication cyst.
Case no 2
A 3-year-old girl presented with history of pain abdomen for 6 months. There was history of viral fever lasting a week prior to this. Abdominal ultrasound done 3 months earlier was reported as pseudocyst of pancreas. Conservative management was being tried in a hope for its natural resolution but the cyst persisted. Since the symptoms did not abate, she was referred to our tertiary referral centre. Serum amylase levels were normal. Ultrasound examination at our institute showed a cystic lesion in relation to body of pancreas. It was 5x5 cm in size and had echogenic lining. This feature led to the diagnostic possibility of gastric duplication cyst (GDC). At exploratory laparotomy ,GDC of 5x6 cm in size was found to be arising from greater curvature of the stomach and was very closely abutting the body of the pancreas. Total excision of GDC was accomplished. The cyst contained clear fluid. Histopathology report confirmed the diagnosis of GDC.
Discussion
GDC may be tubular and cystic.[2] The latter usually does not communicate with the gastric lumen. However, majority of GDC are attached to the wall of stomach, as was found in the cases presented above. Most commonly they are seen along the greater curvature but they may arise from lesser curvature, anterior wall, posterior wall or pylorus. In our first patient GDC was originating from the lesser curvature while in the second patient it was from the greater curvature.
The presentation of GDC may be with vomiting, malena or hemetemesis due to GI hemorrhage, abdominal mass, pain abdomen and failure to thrive. Perforation peritonitis[2] malignant degeneration[3] and chronic pancreatitis[4] are few other complications of these lesions. There may even recur after incomplete resection.[1]
The differential diagnosis of GDC include pseudocyst of pancreas[5], hydatid cyst, omental cyst, pseudocyst of spleen etc. However, the main difficulty arises in differentiating pseudocyst of pancreas from GDC, as happened in both our patients. In the first patient the history of abdominal trauma and an elevated serum amylase level strongly suggested pancreatic pseudocyst whereas, in the second case viral fever was considered as an antecedent event for pancreatitis. A raised serum amylase level in our first case in all likelihood was due to proximity of the GDC to pancreas as there was no sonographic evidence of pancreatic pathology (ductal dilatation, pancreatic tissue edema etc). However, a closer look at past history in the first case should have suggested a long standing illness. In the second case, a normal amylase levels and sonographic finding of echogenic lining in the cyst clinched the diagnosis.
On reviewing literature, we found that psancreatic pseudocyst can pose a real diagnostic dilemma for patients with GDC. Maeda et al described this diagnostic confusion in a 63-yr-old female on account of presence of a thin walled cystic lesion.[6] In fact even endoscopic sonography failed to identify the muscle bundles in the GDC wall in their case. Rullier et al7 also affirm that the distinction between pancreatic pseudocyst and GDC could be very difficult on account of many clinical and radiological similarities. They propose few distinguishing points that can distinguish these lesions preoperatively. On abdominal sonography and CT scan, pancreatic pseudocyst appear as ill defined cystic mass at the tail of pancreas or in lesser sac, with displacement of stomach anteriorly, whereas, the GDC is generally present as a well defined cystic mass in the gastro-duodenal region. Echogenic mucosal lining is specific for GDC.[7,8] Pancreatic enzymes are typically raised in patients with pseudocyst. Also, the features of acute or chronic pancreatitis like necrotic areas in pancreas with ductal dilatation with calcification/fat saponification surrounding thick Gerota fascia are generally present in the patient with PPC and help in clinching the diagnosis. Occasionally Upper GI series (passage of contrast in GDC due to its communication with gastric lumen) and scintigraphy[9] (ectopic mucosa in GDC) help in arriving at the diagnosis MRI may show a thin layer between the cyst and the body of pancreas and the surrounding organs and thus rule out possibility of a lesion being pancreatic pseudocyst.[6]
Conclusion
The possibility of GDC should be kept in children presenting with cystic lesion in upper abdomen, distinguish them from pseudocyst pancreas and other lesions using detailed history and diagnostic investigations.
References
1. Bond SJ, Groff DB. Gastrointestinal duplication. In: O’Neill JA, Rowe MI, Grosfield JL, Fonkalsrud EW, Coran AG, editors. Pediatric surgery. 5th edition. London: Mosby; 1998. p.1257–65.
2. Ratan SK, Ratan J, Lohan A, Roychaudhary R. Unusual presentation of gastric duplication cyst in a neonate with pneumoperitoneum and vertebral anomalies Am J Perinatol. 2002;19:361–6.
3. Kuraoka K, Nakayama H, Kagawa T, Ichikawa T, Yasui W. Adenocarcinoma arising from a gastric duplication cyst with invasion to the stomach: a case report with literature review. J Clin Pathol. 2004;57:428–31.
4. Lavine JE, Harrison M, Heyman MB. Gastrointestinal duplications causing relapsing pancreatitis in children. Gastroenterology. 1989;97:1556–8.
5. Parakh P, Lalwani N, Udawat M. Pseudocyst of pancreas associated with gastric duplication. Ind J Radiol Imag.2002;12:493–5.
6. Maeda H, Okabayashi T, Nishimori I,Kobayashi M, Morimoto K, Miyaji E, et al. Diagnostic challenge to distinguish gastric duplication cyst from pancreatic cystic lesions in adult. Intern Med. 2007;46:1101–4.
7. Rullier E, Carles J, Zerbib F, Chaussende C, Saric J. Gastric duplication or pancreatic pseudocyst. Diagnostic difficulties apropos of a case. Gastroenterol Clin Biol. 1996;20:200–3.
8. Kangarloo H, Sample WF, Hansen G, Robinson JS, Sarti D. Ultrasonic evaluation of abdominal gastrointestinal tract duplication in children. Radiology. 1979;131:191–4.
9. Dittrich JR, Spottswood SE, Jolles PR. Gastric duplication cyst. Scintigraphy and correlative imaging. Clin Nucl Med. 1997;22:93–6.
|