48uep6bbphidvals|344
48uep6bbph|2000F98CTab_Articles|Fulltext
Characterization of focal liver lesions remains a diagnostic challenge for the radiologists, more so when there is associated underlying chronic liver disease (CLD). Imaging plays a vital role and there has been a constant endeavor to improve the diagnostic accuracy of liver lesions. The introduction of mutiphasic CT and MRI has revolutionized the diagnostic ability of liver lesions. With the advent of ultrasound contrast agents (UCAs), it is possible to evaluate liver lesions using the non-invasive imaging technique of “contrast-enhanced ultrasonography” (CEUS).[1,2,3] CEUS can overcome the limitations of grey scale and color doppler sonography[4,5] and has been used for characterisation of focal liver lesions.[2]. It can depict arterialisation of hypervascular hepatocellular carcinoma (HCC)[6,7] and can also help in assessment of the post-therapeutic response.[8,9]
We present two such cases of HCC evaluated by a recently available second generation ultrasound contrast agent, SonoVue (Bracco, UK).
Technique of CEUS
Initial grey scale ultrasound (US) was performed to look for a proper acoustic window, visibility of lesion and patient co-operation. CEUS was performed on Siemens S-2000 machine. The vial of SonoVue was prepared 5 minutes prior to CEUS by injecting 5 ml of saline into the powdered form of the vial and shaking vigorously. An intravenous antecubital access with 20-gauge venflon with a three-way connector was obtained.
After selecting contrast specific imaging mode, 2.4 ml of SonoVue per mass was injected intravenously followed by normal saline flush of 10 ml. The timer was started immediately following the contrast injection and findings recorded on cine mode. Enhancement of the mass was evaluated in three phases - arterial (15-25 seconds), portal venous (45-90 seconds) and delayed phase (180 seconds).The cine recordings were reviewed later and the pattern and peak enhancement of the mass in each phase was noted.
Case 1
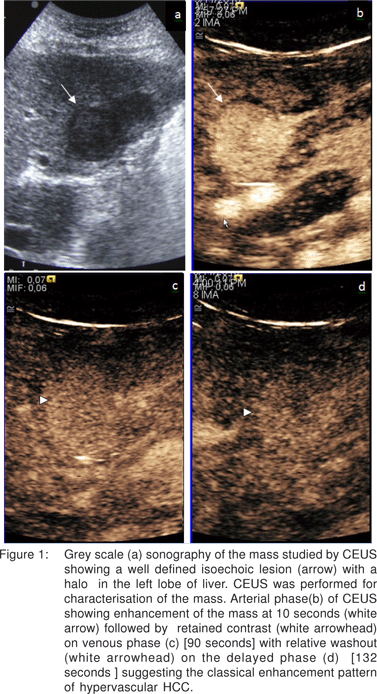
A 45 years old female, known case of hepatitis C associated CLD presented with ascites. She was found to have deranged liver function tests. Serum a-feto protein was markedly elevated, 4392 ng/ml. US showed multiple focal, hypoechoic lesions in left lobe of liver, the largest one was isoechoic with a halo around and measuring 2.8 x 2.4 cm [Figure 1a].
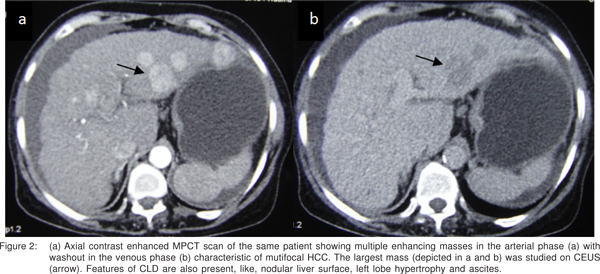
CEUS was done for the largest mass.It showed intense enhancement on the arterial phase, retained contrast in the venous phase with relative washout in the delayed phase [Figure 1bd], depicting the characteristic pattern of hypervascular HCC in the setting of CLD. MPCT confirmed the findings of HCC (Figure 2a-b).
Case 2
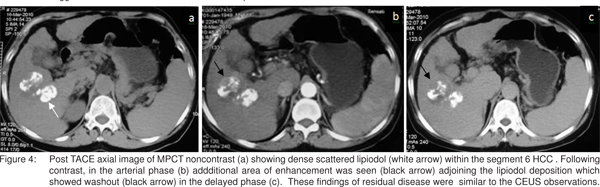
A 61 years old female, known case of hepatitis B related CLD with solitary large HCC who was treated with TACE was subjected to CEUS at one month following TACE. Grey scale ultrasound showed a large heterogenous HCC (4.5 X 2.5cm) in segment 6 (hyperechoic area superiorly and remaining hypoechoic) (Figure 3 a). CEUS showed an area of nodular enhancement superiorly within the mass in the arterial phase with washout in the delayed phase suggesting residual disease.The remaining tumor was non-enhancing [Figure 3 b,c]. MPCT confirmed the findings of CEUS. [Figure 4a-c].
Discussion
Imaging plays a vital role in the evaluation of liver lesions. With the advent of multidetector CT and newer MR sequences, multiphasic evaluation of liver is feasible, thus increasing sensitivity and specificity of the diagnosis of liver lesions. Another recent advance in this field is the introduction of CEUS.
Grey scale sonography is the first imaging modality for the evaluation of liver and widely recommended for surveillance of high risk patients for HCC detection.[10,11] However, gray scale and color doppler sonography have numerable limitations.[5] In order to improve the diagnostic ability, UCAs were developed. UCAs are microbubbles stabilized with galactose-palmitic acid or phospholipid shell containing either air (first generation agents) or other inert gases such as sulphur hexafluoride (second generation contrast agents). UCAs are blood pool agents and act by increasing the backscatter by virtue of its non-linear wide band harmonic response at low mechanical index[12] and facilitates better evaluation of liver lesions, compared with gray scale alone.[2,13,14,15]. The only contrast agent available in India is, SonoVue, Bracco (UK). SonoVue contains sulphur hexafluoride stabilized by phospholipid shell. It is a pure blood pool agent with no equilibrium phase. These features make SonoVue an ideal contrast agent for vascular phase (arterial, venous and delayed phase) imaging of liver lesions.[1,12]

The UCAs are safe and have few non specific side effects resolving spontaneously.[10,12] Rarely life threatening anaphylactoid reactions can occur which mandate precautionary measures to be ready. They can be safely used in patients with renal failure.[10] Sonazoid (GE Healthcare, Milwaukee, USA), another UCA, is available exclusively in Japan. Sonazoid differs from other agents in exhibiting post vascular phase till 60 to 120 minutes and helps in better lesion characterization.[16]
CEUS has high sensitivity in the detection and characterisation of liver lesions with diagnostic accuracy comparable to multiphasic CT.[6,7,8] It is useful for differentiating benign from malignant focal liver lesions[17] and exhibits classical enhancement patterns facilitating better characterization.[5,8,18] Guidance of percutaneous ablative therapy for lesions not detected by gray scale sonography can also be achieved by CEUS.[19] Additionally, the assesment of treatment response in patients of HCC who have undergone tranarterial chemoembolization (TACE),radiofrequency ablation ( RFA) or percutaneous acetic acid injection is also possible.[8,9]
The classical diagnosic pattern for HCC on multiphasic CT and MRI is the presence of enhancement in the arterial phase with washout of the contrast in venous or delayed phase. CEUS could successfully diagnose hypervascular HCC and depict the residual disease as nodular arterial enhancement with nonenhancing lipiodol very well in the two above cases, scoring over grey scale sonography on which this detection is not possible. Multiplanar computed tomography (MPCT) confirmed the findings of CEUS in both the cases of HCC.
To conclude, CEUS is a simple, safe and promising imaging modality for characterization of focal liver lesions and for assessment of post treatment response. Larger studies are needed to evaluate the diagnostic accuracy of this new imaging technique.
References
1. Soye JA, Mullan CP, Porter S, Beattie H, Barltrop AH, Nelson WM. The use of contrast-enhanced ultrasound in the characterisation of focal liver lesions. Ulster Med J. 2007;76:22– 5.
2. Quaia E, Calliada F, Bertolotto M, Rossi S, Garioni L, Rosa L, et al. Characterization of focal liver lesions with contrast-specific US modes and a sulphur hexafluoride-filled microbubble contrast agent: diagnostic performance and confidence. Radiology. 2004;232:420–30.
3. Isozaki T, Numata K, Kiba T, Hara K, Morimoto M, Sakaguchi T, et al. Differential diagnosis of hepatic tumors by using contrast enhancement patterns at US. Radiology. 2003;229:798–805.
4. Wilson SR, Burns PN, Muradali D, Wilson JA, Lai X. Harmonic hepatic US with microbubble contrast agent: initial experience showing improved characterization of hemangioma, hepatocellular carcinoma, and metastasis. Radiology. 2000;215:153–61.
5. Leen E. The role of contrast-enhanced ultrasound in the characterization of focal liver lesions. Eur Radiol. 2001;11(suppl 3):E27–34.
6. Numata K, Tanaka K, Kiba T, Saito S, Ikeda M, Hara K, et al. Contrast-enhanced,wide-band harmonic grayscale imaging of hepatocellular carcinoma: correlation with helical computed tomographic findings. J Ultrasound Med. 2001;20:89–98.
7. Burns PN, Wilson SR. Focal liver masses: Enhancement patterns on contrast-enhanced images— concordance of US scans with CT scans and MR images. Radiology. 2007;242:162–74.
8. Ding H, Kudo M, Onda H, Suetomi Y, Minami Y, Chung H, et al. Evaluation of posttreatment response of hepatocellular carcinoma with contrast-enhanced coded phase-inversion harmonic US: comparison with dynamic CT. Radiology. 2001;221:721–30.
9. Wen YL, Kudo M, Zheng RQ, Minami Y, Chung H, Suetomi Y, et al. Radiofrequency ablation of hepatocellular carcinoma: therapeutic response using contrast-enhanced coded phaseinversion harmonic sonography. AJR Am J Roentgenol. 2003;181:57–63.
10. Lencioni R, Piscaglia F, Bolondi L. Contrast-enhanced ultrasound in the diagnosis of hepatocellular carcinoma. J Hepatol. 2008;48(5):848–57.
11. Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–36.
12. Claudon M, Cosgrove D, Albrecht T, Bolondi L, Bosio M, Calliada F, et al. (EFSUMB study group). Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS)- update 2008. Ultraschall Med. 2008;29:28– 44.
13. Leen E, Angerson WJ, Yarmentis S, Bongartz G, Blomley M, Del Maschio A, et al. Multi-centre clinical study evaluating the efficacy of SonoVue (BR1), a new ultrasound contrast agent in Doppler investigation of focal hepatic lesions. Eur J Radiol. 2002;41:200–6.
14. Quaia E, Bertolotto M, Calderanl L, Mosconi E, Mucelli RP. US characterization of focal hepatic lesions with intermittent highacoustic- power mode and contrast material. Acad Radiol. 2003;10:739–50.
15. Nicolau C, Catala V, Vilana R, Gilabert R, Bianchi L, Sole M, et al. Evaluation of hepatocellular carcinoma using SonoVue, a second generation ultrasound contrast agent: correlation with cellular differentiation. Eur Radiol. 2004;14:1092–9.
16. Kudo M. The 2008 Okuda lecture. Management of hepatocellular carcinoma: from surveillance to molecular targeted therapy. J Gastroenterol Hepatol. 2010;25:439–52.
17. Von Herbay A, Westerndorff J, Gregor M. Contrast-enhanced ultrasound with SonoVue: differentiation between benign and malignant focal liver lesions in 317 patients. J Clin Ultrasound. 2010;38:1–9.
18. Ricci P, Laghi A, Cantisani V, Paolantonio P, Pacella S, Pagliara E, et al. Contrast-enhanced songraphy with SonoVue: enhancement patterns of benign focal liver lesions and correlation with dynamic gadobenate dimeglumine-enhanced MRI. AJR Am J Roentgenol. 2005;184:821–7.
19. Numata K, Morimoto M, Ogura T, Sugimori K, Takebayashi S, Okada M, et al. Ablation therapy guided by contrast-enhanced sonography with Sonazoid for hepatocellular carcinoma lesions not detected by conventional sonography. J Ultrasound Med. 2008;27:395–406.