48uep6bbphidvals|382
48uep6bbph|2000F98CTab_Articles|Fulltext
Introduction
Hepatic venous outflow tract obstruction (HVOTO) refers to a variety of disorders causing obstruction of hepatic venous outflow or suprahepatic inferior vena cava (IVC) or both resulting in increased hepatic sinusoidal pressure and portal hypertension.[1] Acute hepatic venous thrombosis is the most common cause for HVOTO in west, and is largely associated with oral contraceptive pills, pregnancy or underlying thrombotic disorders.[2,3,4,5,6,7] In contrast, membranous/fibrous obstruction of IVC, hepatic vein or both is more frequent in the Indian subcontinent.[8,9,10,11] Most patients with HVOTO due to isolated suprahepatic IVC obstruction have been reported to have membranous or fibrotic obstruction of IVC.[12] Earlier membranous obstruction of IVC and/or HV was considered to be congenital, studies from Japan documented that these webs and membranes were consequence of organized thrombus developing insidiously[13] which is likely to be the pathogenic process in patients with HVOTO presenting with insidious onset of the disease.
Predominant clinical manifestations of HVOTO include, ascites usually containing high protein, and hepatomegaly with endoscopic, clinical and radiological evidence of portal hypertention. Often such patients may initially manifest with variceal bleeding, and splenomegaly with or without ascites. Pedal edema, chronic pigmentation of legs, varicosity of veins in lower extremities may accompany above features. Surgical porto-systemic shunting was considered to be conventional treatment for patients having HVOTO with preserved liver functions whereas liver transplantation was reserved for those with extremely poor liver function due to end stage chronic liver disease or for those presenting with clinical syndrome of acute liver failure.[14,15,16,17,18,19] However recent studies and clinical trials have documented encouraging midterm outcome subsequent to radiological interventions resulting in recanalization of stenosed segment of hepatic vein and/or IVC or creation of transjugular intrahepatic portosystemic shunt (TIPS).[20,21,22,23,24,25,26,27] Restoring normal hepatic venous outflow by opening/dilating one of the stenosed HV and/or IVC is considered the most physiological way of treating HVOTO. Patients in whom negotiation of hepatic vein through vena cava is not possible, percutaneous dilatation of these veins has also been attempted.
However, such attempts are usually red undant when all the three hepatic veins are completely obliterated throughout their length and are replaced with multiple collaterals. However, this subset of patients has been successfully treated with TIPS for alleviation of symptoms. In this article we will be discussing the various radiological procedures performed in patients with HVOTO and also provide the results of efficacy of such interventions.
Etio-pathogenesis and clinical manifestations
HVOTO usually occurs in young adults and most of the patients have been reported to have hematologic abnormalities causing prothrombotic states. Amongst various thrombotic disorders myeloproliferative disease is the most frequently described association in patients with HVOTO.27 Other common causes include JAK2 V617F mutation (also associated with myeloproliferative abnormality), Factor V Leiden mutation, oral contraceptive pills, pregnancy, antiphospholipid antibodies, paroxysmal nocturnal hemoglobinuria, and inherited protein C and S deficiency (necessary for anticoagulation effect).[27] There may be presence of multiple causes in an individual patient. HVOTO may clinically present as acute and fulminant forms or as chronic disease. IVC and HV obstruction due to web/ membrane presenting in a chronic form is more frequent in the Indian subcontinent.[8,9,10,11] Few Indian studies have also documented prevalence of hypercoagulable state in Indian patients and reported certain change in spectrum of VOTO in India.[28,29] One of these studies highlighted that HV thrombosis may be more frequent than IVC thrombosis/web which is in contrast to other previous Indian studies.[28] However, thesestudies[28] include small number of cases and were reported from tertiary care referral centre which might have resulted in such bias.
Secondary HVOTO may be seen in patients having primary kidney, liver, suprarenal gland tumors with metastatic deposits compressing the IVC and HV. Tumors of IVC and heart also constitute rare causes of HVOTO. The basic abnormality in HVOTO is the obstruction of hepatic vein or suprahepatic IVC above or at the opening of HVs to IVC, which leads to increase in sinusoidal pressure and hepatic congestion consequent to which portal hypertension develops. Increased sinusoidal pressure causes reduction in hepatic perfusion further leading to ischemia of hepatocytes and its necrosis in perivenular zones. These changes lead to perivenular hepatic fibrosis, nodular regenerative hyperplasia and ultimately cirrhosis.
Clinical manifestation usually depends upon site of obstruction, rapidity with which the obstruction in hepatic venous outflow develops and ability of consequent collaterals to decompress the obstructed segments. Therefore HVOTO patients may be asymptomatic, present with features of portal hypertension, may manifest with features of liver failure associated with chronic parenchymatous liver disease or may even present with clinical syndrome of acute liver failure requiring urgent intervention.
Imaging and interventions
Various imaging modalities used in the evaluation of HVOTO include ultrasound (US) and color Doppler examination, computed tomography (CT) and magnetic resonance imaging (MRI). HVOTO is associated with variable imaging findings depending upon the rapidity with which the disease manifests (acute or chronic). In acute setting liver is enlarged with thrombosed hepatic veins. In chronic forms of the disease imaging findings range from IVC and/or HV obstruction, either due to web or short segment fibrotic stenosis, to more pronounced long segment involvement of HV, to complete replacement of native hepatic veins by collaterals. Venous collaterals which are termed as ‘comma shaped’ collaterals within the liver are an essential finding on imaging to diagnose HVOTO. Other imaging findings correspond to degree of hepatic fibrosis, nodular regenerative hyperplasia, cirrhosis and presence of portal hypertension.
US and color Doppler are the most widely used imaging modalities for evaluating HVOTO, both for treatment planning and for follow up after radiological intervention. It allows easy assessment of hepatic veins, IVC, liver parenchyma and associated collateral venous channels (Figure 1). CT and MRI play a supplemental role and depict obstruction or patency of HV and/or IVC and may help in differentiating IVC compression due to hypertrophied caudate lobe from IVC occlusion due to fibrotic stenosis/membrane (Figure 2). These modalities also identify if superimposed secondary thrombosis is present which may cause an acute manifestation of previously silent fibrotic occlusion of hepatic venous outflow tract.
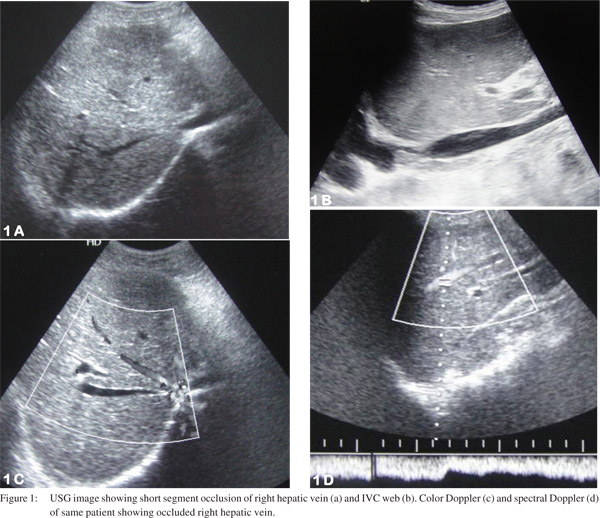
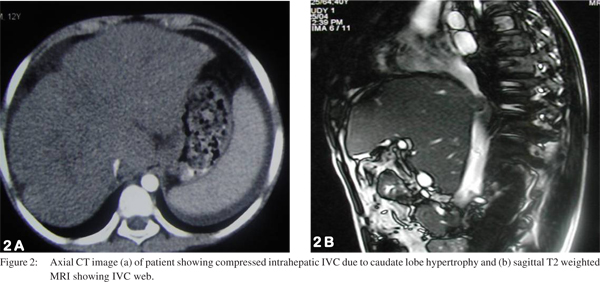
Over the last decade various studies[20,21,22,23,24,25,26,27] (Table 1) have shown effectiveness and good outcome following various radiological interventions in HVOTO patients and recently therehas been a consensus over the management consisting of minimally invasive technique over surgical procedures.0 [20,21,22,23,24,25,26,27] Restoring hepatic venous outflow and opening of IVC occlusion is the preferred treatment in HVOTO if technically feasible. Other treatment option is creation of TIPS and surgical options are reserved for patients with failed radiological interventions and fulminant disease with liver failure and are usually recommended for liver transplantation. Algorithm for the management of HVOTO is provided as flowchart (Figure 3).

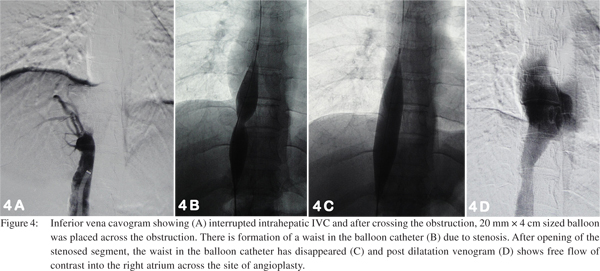
For planning interventions in patients having chronic HVOTO, patients are categorized into two subsets. First group consists of patients having short segment/membranous occlusion of HV/IVC or both. The second group consists of patients with all the native hepatic veins totally replaced by collaterals and the visible veins being too small and having insufficient flow, which is not suitable for interventions aimed at restoring normal physiological hepatic venous outflow. Treatment
for the first group consists of opening of the blocked segment of HV
and/or IVC by transjugular, transfemoral or percutaneous route. The
second group of patients requires creation of non-surgical portocaval
shunt (TIPS) and in case of severe liver failure (end stage liver
disease or those presenting with features of acute liver failure)
ultimate treatment remains liver transplantation. Any radiological
intervention should always be supplemented with hematological management
as well, because in absence of hematological management there is a very
high likelihood of treatment failure, in the form of restenosis of
recanalized hepatic venous outflow or thrombosis of TIPS.
IVC and hepatic venous interventions
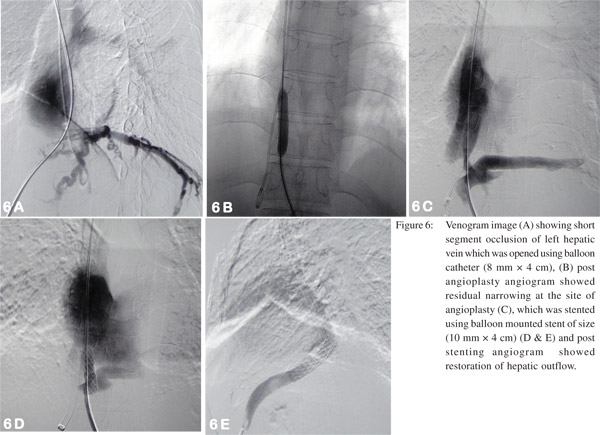
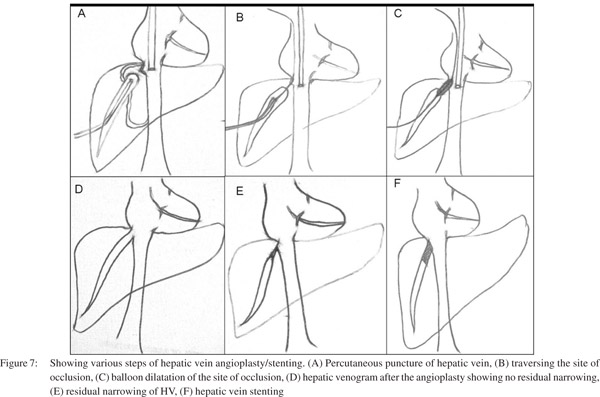
Patients with IVC involvement may have isolated suprahepatic IVC occlusion or intrahepatic IVC occlusion just above the confluence with hepatic veins. Management of these patients consists of balloon angioplasty (dilatation) of the stenosed segment or in few cases vascular metallic stents may be required. Initially a guide wire is negotiated across the web/ stricture then serial dilatation of stenosed segment is done using a balloon catheter (Figure 4). If stricture persists even after dilatation with a balloon having equal size as of lumen of adjacent normal segment of IVC, then the stent should be placed, however most of the strictures open up completely following adequate dilatation. These patients have fairly good midterm outcome with immediate relief from symptoms like pedal edema, dilated veins over the trunk and varicose veins. In patients having hepatic venous occlusion, the healthiest HV is selected for dilatation. Generally veins having straight course, good caliber (7-8 mm in young adults) and an echofree lumen is considered healthy and suitable for interventions. Various approaches are described to cross the occluded segment which include transjugular, percutaneous transhepatic and combined. Once the stricture is crossed, angioplasty of the stenosed segment if performed and stenting may be done if required (Figure 5). Decision of stent placement depends upon the post dilatation status of the occluded segment of the hepatic vein. Persistence of stenosis suggests that stent is required to maintain the patency (Figure 6).[30, 31]

Patients having occlusion of both IVC as well as HV require dilatation of both hepatic vein and IVC and decision of stent placement depends on post procedure venogram. Some of these patients are difficult to treat as they may have a very tight fibrosis causing closure of HV, IVC or both. In such cases multiple approaches via all three transjugular, transfemoral and percutaneous transhepatic may be needed. At times Chiba needle may be needed to cross these very tight fibrotic strictures, but use of the needle should be always done with a great degree of caution and by an experienced interventional radiologist, as even the slightest mistake may be fatal. Even in acute Budd-Chiari syndrome (BCS) radiological intervention has some role in selected patients who may need local/direct pharmacological and/or mechanical thrombolysis to restore deteriorating liver functions. However, some case reports have described benefits of systemic thrombolysis, whereas few investigators have used combined local and systemic thrombolysis.[32,33] Local direct pharmacological as well as mechanical thrombolysis may benefit patients with fulminant disease not responding to systemic thrombolysis and anticoagulation. One study has shown the role of local pharmacological thrombolysis in acute cases or patients presenting recent post procedure thrombosis of stenosed segment/stent.[34]
TIPS (Transjugular intrahepatic portosystemic shunt)
Interventional management for other group of patients of chronic BCS with all hepatic veins replaced by thin collaterals is usually treated by TIPS. Now-a-days TIPS is one of the most common intervention performed in patients with BCS in west, but it is less commonly performed in india. However there are reports from some centres which have reported good short term outcome.[27,28,35,36,37,38,39,40,41] Performing TIPS in a patient with BCS may be technically challenging as there is no patent hepatic vein available for creation of shunt. Sometimes even the stump/ notch of occluded hepatic vein is also not seen. In these cases some modification is made in the technique and a direct portocaval shunt is established. Generally the procedure is performed via transjugular route but in few cases combined percutaneous transhepatic approach may be needed for creation of shunt. Some authors have even described the successful performance of percutaneous transhepatic ultrasound guided TIPS.[42] Successful TIPS relieves the liver congestion and hence leads to improved clinical symptoms and liver function of the patient along with arrest in the ongoing liver fibrosis.
Equipment and technique
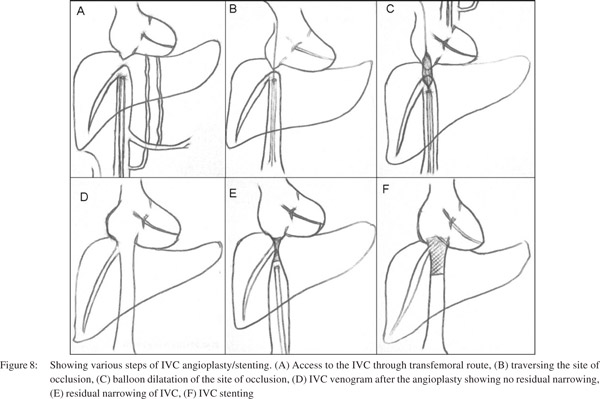
Technique of IVC angioplasty
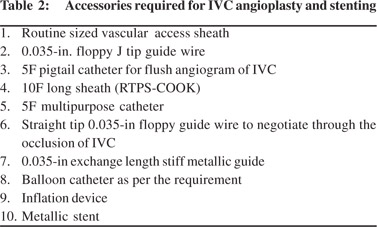
IVC angioplasty (Figure 7) may be performed either by transjugular or transfemoral approach, however the transfemoral approach is preferred. The transfemoral approach provides a straight pathway and easy as well as comfortable catheter control and manipulation to negotiate the occluded segment as compared to transjugular approach. At the onset, a wide bore (9-10F) long sheath should be placed inside the IVC, which is of great help. It provides adequate strength to the coaxial angiographic catheter and guide wire combination for difficult manipulation and also a larger size balloon (16- 20mm) can be used through this long sheath. Having placed a long sheath in the IVC the occluded segment should be negotiated using straight tip floppy end guide wire and angiographic catheter combination. Occasionally, if the occluded segment is resistant to negotiation using the floppy end, then hard end of the guide wire may be used, but with caution. Hard end of guide wire must be used with great care and by experienced hands. After negotiating the occlusion, floppy guide wire is exchanged with stiff metallic guide wire. At this stage patient should be administered additional bolus of heparin (50 IU x patient’s body weight) should be given. Stenotic segment is then dilated initially by smaller diameter balloon catheter followed by a larger balloon catheter. If there is no significant gradient across the stenotic segment, the procedure is supposed to be completed. Common accessories required for this procedure is listed in Table 2.
Technique of HV angioplasty and stenting
HV angioplasty (Figure 8) is generally performed by transjugular route however percutaneous transhepatic route may be required in some patients whereas few patients may require combination of both approaches. The approach depends upon the operator’s preference and experience. Each approach has its own advantages. Through transjugular route initially a venogram is performed. If hepatic vein stump is seen, attempt should be made to negotiate the site of occlusion using either guide wire-catheter combination (placed coaxially within the long sheath) or by Colapinto needle. If there is difficulty in negotiating the site of occlusion through transjugular route, percutaneous transhepatic route may be used. Under USG guidance, hepatic vein is punctured, cannulated and venogram is performed to obtain baseline image to depict the exact site of occlusion. Using catheter-guide wire combination, the site of occlusion is negotiated. The negotiated guide wire is snared through the transjugular route, however if this is not possible then the wire should be sent as distal as possible to park it in a distal peripheral vein. Later the floppy guide wire is exchanged with exchange length stiff metallic guide wire and the stenotic segment is dilated with an appropriate sized balloon. At this stage additional bolus of heparin (50 IU x patient’s body weight) should be given. If there is no significant gradient across the stenotic segment, the procedure is complete. However, if there is residual stenosis, stenting should be considered (balloon mounted stent is preferred over self expanding stent as balloon mounted stent can be positioned more perfectly as compared to self expandable metalic stent). If the procedure is performed percutaneously then the tract should be embolised using embolisation coils and gel-foam to prevent hemoperitonium and the patient should be followed after the procedure for any intra-abdominal bleed under close observation. Table 3 provides the list of accessories commonly needed in this procedure.
Technique of TIPS)
TIPS is usually performed by transjugular route, however modified combined percutaneous transhepatic and transjugular approach (Figure 9). The 10 F, 40 cm long vascular sheath is introduced into IVC through the right transjugular route. The stump of the occluded right hepatic vein is used for the access to traverse the hepatic parenchyma. The Colapinto needle is rotated anteriorly and advanced parallel to the spine to the anticipated location of intrahepatic portal vein. Caution is exercised to avoid capsular transgression. The syringe is aspirated during slow needle withdrawal. When blood is aspirated, contrast is injected to identify the vascular structure entered. If the suitable branch of intrahepatic portal vein (2 cm beyond the portal bifurcation) has been punctured, the Terumo guide wire is manipulated though the main portal vein into superior mesenteric vein. Hand injected portography is obtained. The parenchymal tract is dilated with 8 and 10 mm angioplasty balloons. Later the entire tract is covered with stent graft of appropriate length, measured using catheter with markers. The uncovered portion of the stent (2 cm) is placed inside the portal vein. Rest of the tract is covered with the covered portion of stent. All the access catheters are removed after shunt formation. Common hardware needed in this procedure is listed in Table 4.
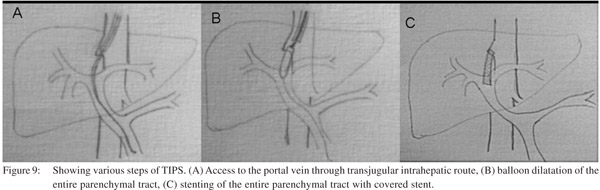

Role of surgery
Due to availability of various non invasive radiological interventions and techniques, with a good midterm outcome, shunt surgery has lost its place in the management of BCS. Liver transplantation has a definite role in treating patients having end stage liver disease or with fulminant disease and is the ultimate treatment and salvage procedure.
Follow up and post intervention management
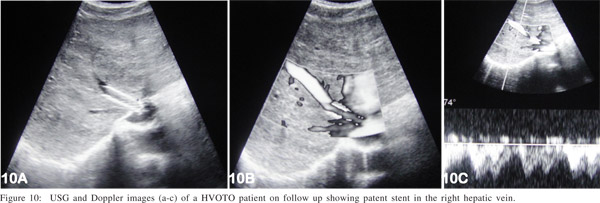
Post intervention management is the key for long term success of the procedure. Most of the patients of BCS have underlying thrombotic disorder(s) and hypercoagulable state, so it is very important to maintain INR (international normalized ratio) around 2.5; initially by injectable anticoagulants (heparin) and later by oral anticoagulants (warfarin). In the absence of anticoagulation there is a very high likelihood of restenosis/ thrombosis of recanalized segment/stent. Regular clinical and radiological follow up is equally important. Initially the patient is reviewed every three months for a year; thereafter annual follow up is done. Radiological follow up consists of ultrasound and color Doppler evaluation of liver and recanalized HV/IVC (Figure 10) and of stent graft in case of TIPS. Whenever there is a doubt hepatic venogram and inferior vena cavogram is performed. If there is any suggestion of restenosis either due to thrombus or intimal hyperplasia, balloon dilatation is done for the same and decision of stent placement depends on post dilatation venogram. If deemed necessary restenting may be undertaken.
Conclusion
BCS refers to a heterogeneous group of patients having hepatic venous outflow obstruction and/or IVC occlusion leading to raised hepatic sinusoidal pressure, hepatic fibrosis and portal hypertension. Radiological intervention has emerged as the primary treatment for this disorder consisting of recanalization, balloon dilatation, stent placement and creation of portosystemic shunt. With various studies showing good mid-term outcome following radiological interventions in patients of BCS, shunt surgery has lost its place as a primary treatment modality. However liver transplant remains the ultimate treatment and salvage procedure in selected cases.
References
1. Ludwig J, Hashimoto E, McGill DB, van Heerden JA. Classification of hepatic venous outflow obstruction: ambiguous terminology of the Budd-Chiari syndrome. Mayo Clin Proc. 1990;65:51–5.
2. Hadengue A, Poliquin M, Vilgrain V, Belghiti J, Degott C, Erlinger S, et al. The changing scene of hepatic vein thrombosis: recognition of asymptomatic cases. Gastroenterology. 1994;106:1042–7.
3. Denninger MH, Chait Y, Casadevall N, Hillaire S, Guillin MC, Bezeaud A, et al. Cause of portal or hepatic venous thrombosis in adults: the role of multiple concurrent factors. Hepatology. 2000;31:587–91.
4. Mohanty D, Shetty S, Ghosh K, Pawar A, Abraham P. Hereditary thrombophilia as a cause of Budd-Chiari syndrome: a study from Western India. Hepatology. 2001;34:666–70.
5. Valla D, Le MG, Poynard T, Zucman N, Rueff B, Benhamou JP. Risk of hepatic vein thrombosis in relation to recent use of oral contraceptives. A case-control study. Gastroenterology. 1986;90:807–11.
6. Valla DC. The diagnosis and management of the Budd-Chiari syndrome: consensus and controversies. Hepatology. 2003;38:793–803.
7. Rosenberg RD, Aird WC. Vascular-bed-specific hemostasis and hypercoagulable states. N Engl J Med. 1999;340:1555–64.
8. Kohli V, Pande GK, Dev V, Reddy KS, Kaul U, Nundy S. Management of hepatic venous outflow obstruction. Lancet. 1993;342:718–22.
9. Dilawari JB, Bambery P, Chawla Y, Kaur U, Bhusnurmath SR, Malhotra HS, et al. Hepatic outflow obstruction (Budd-Chiari syndrome). Experience with 177 patients and a review of the literature. Medicine (Baltimore). 1994;73:21–36.
10. Singh V, Sinha SK, Nain CK, Bambery P, Kaur U, Verma S, et al. Budd-Chiari syndrome: our experience of 71 patients. J Gastroenterol Hepatol. 2000;15:550–4.
11. De BK, De KK, Sen S, Biswas PK, Das TK, Das S, et al. Etiology based prevalence of Budd-Chiari syndrome in eastern India. J Assoc Physicians India. 2000;48:800–3.
12. Victor S, Jayanthi V, Madanagopalan N. Coarctation of the inferior vena cava. Trop Gastroenterol. 1987;8:127–42.
13. Okuda K. Inferior vena cava thrombosis at its hepatic portion (obliterative hepatocavopathy). Semin Liver Dis. 2002;22:15–26.
14. Orloff MJ, Girard B. Treatment of Budd-Chiari syndrome by side-to-side portacaval shunt: long-term results. Hepatology. 1986;6:1181.
15. Halff G, Todo S, Tzakis AG, Gordon RD, Starzl TE. Liver transplantation for the Budd-Chiari syndrome. Ann Surg. 1990;211:43–9.
16. Klein AS, Sitzmann JV, Coleman J, Herlong FH, Cameron JL. Current management of the Budd-Chiari syndrome. Ann Surg. 1990;212:144–9.
17. Panis Y, Belghiti J, Valla D, Benhamou JP, Fekete F. Portosystemic shunt in Budd-Chiari syndrome: long-term survival and factors affecting shunt patency in 25 patients in Western countries. Surgery. 1994;115:276–81.
18. Tilanus HW. Budd-Chiari syndrome. Br J Surg. 1995;82:1023–30..
19. Hemming AW, Langer B, Greig P, Taylor BR, Adams R, Heathcote EJ. Treatment of Budd-Chiari syndrome with portosystemic shunt or liver transplantation. Am J Surg. 1996;171:176–80; discussion 80–1.
20. Fisher NC, McCafferty I, Dolapci M, Wali M, Buckels JA, Olliff SP, et al. Managing Budd-Chiari syndrome: a retrospective review of percutaneous hepatic vein angioplasty and surgical shunting. Gut. 1999;44:568–74.
21. Wu T, Wang L, Xiao Q, Wang B, Li S, Li X, et al. Percutaneous balloon angioplasty of inferior vena cava in Budd-Chiari syndrome-R1. Int J Cardiol. 2002;83:175–8.
22. Baijal SS, Roy S, Phadke RV, Agrawal DK, Kumar S, Choudhuri G. Management of idiopathic Budd-Chiari syndrome with primary stent placement: early results. J Vasc Interv Radiol. 1996;7:545–53.
23. Zhang CQ, Fu LN, Xu L, Zhang GQ, Jia T, Liu JY, et al. Longterm effect of stent placement in 115 patients with Budd-Chiari syndrome. World J Gastroenterol. 2003;9:2587–91.
24. Li T, Zhai S, Pang Z, Ma X, Cao H, Bai W, et al. Feasibility and midterm outcomes of percutaneous transhepatic balloon angioplasty for symptomatic Budd-Chiari syndrome secondary to hepatic venous obstruction. J Vasc Surg. 2009;50:1079–84.
25. Senzolo M, Cholongitas EC, Patch D, Burroughs AK. Update on the classification, assessment of prognosis and therapy of Budd- Chiari syndrome. Nat Clin Pract Gastroenterol Hepatol. 2005;2:182–90.
26. Eapen CE, Velissaris D, Heydtmann M, Gunson B, Olliff S, Elias E. Favourable medium term outcome following hepatic vein recanalisation and/or transjugular intrahepatic portosystemic shunt for Budd Chiari syndrome. Gut. 2006;55:878–84.
27. Darwish Murad S, Plessier A, Hernandez-Guerra M, Fabris F, Eapen CE, Bahr MJ, et al. Etiology, management, and outcome of the Budd-Chiari syndrome. Ann Intern Med. 2009;151:167–75.
28. Amarapurkar DN, Punamiya SJ, Patel ND. Changing spectrum of Budd-Chiari syndrome in India with special reference to nonsurgical treatment. World J Gastroenterol. 2008;14:278–85.
29. Eapen CE, Mammen T, Moses V, Shyamkumar NK. Changing profile of Budd Chiari syndrome in India. Indian J Gastroenterol. 2007;26:77–81.
30. Witte AM, Kool LJ, Veenendaal R, Lamers CB, van Hoek B. Hepatic vein stenting for Budd-Chiari syndrome. Am J Gastroenterol. 1997;92:498–501.
31. Pelage JP, Denys A, Valla D, Sibert A, Sauvanet A, Belghiti J, et al. Budd-Chiari syndrome due to prothrombotic disorder: midterm patency and efficacy of endovascular stents. Eur Radiol. 2003;13:286–93.
32. Hauser AC, Brichta A, Pabinger-Fasching I, Jager U. Fibrinolytic therapy with rt-PA in a patient with paroxysmal nocturnal hemoglobinuria and Budd-Chiari syndrome. Ann Hematol. 2003;82:299–302.
33. Alioglu B, Avci Z, Aytekin C, Mercan S, Ozcay F, Kurekci E, et al. Budd-Chiari syndrome in a child due to a membranous web of the inferior vena cava resolved by systemic and local recombinanttissue plasminogen activator treatment. Blood Coagul Fibrinolysis. 2006;17:209–12.
34. Sharma S, Texeira A, Texeira P, Elias E, Wilde J, Olliff SP. Pharmacological thrombolysis in Budd Chiari syndrome: a single centre experience and review of the literature. J Hepatol. 2004;40:172–80.
35. Ochs A, Sellinger M, Haag K, Noldge G, Herbst EW, Walter E, et al. Transjugular intrahepatic portosystemic stent-shunt (TIPS) in the treatment of Budd-Chiari syndrome. J Hepatol. 1993;18:217–25.
36. Blum U, Rossle M, Haag K, Ochs A, Blum HE, Hauenstein KH, et al. Budd-Chiari syndrome: technical, hemodynamic, and clinical results of treatment with transjugular intrahepatic portosystemic shunt. Radiology. 1995;197:805–11.
37. Perello A, Garcia-Pagan JC, Gilabert R, Suarez Y, Moitinho E, Cervantes F, et al. TIPS is a useful long-term derivative therapy for patients with Budd-Chiari syndrome uncontrolled by medicaltherapy. Hepatology. 2002;35:132–9.
38. Carnevale FC, Caldas JG, Maksoud JG. Transjugular intrahepatic sportosystemic shunt in a child with Budd-Chiari syndrome: technical modification and extended followup. Cardiovasc Intervent Radiol. 2002;25:224–6.
39. Mancuso A, Fung K, Mela M, Tibballs J, Watkinson A, Burroughs AK, et al. TIPS for acute and chronic Budd-Chiari syndrome: a single-centre experience. J Hepatol. 2003;38:751–4.
40. Olliff SP. Transjugular intrahepatic portosystemic shunt in the management of Budd Chiari syndrome. Eur J Gastroenterol Hepatol. 2006;18:1151–4.
41. Beckett D, Olliff S. Interventional radiology in the management of Budd Chiari syndrome. Cardiovasc Intervent Radiol. 2008;31:839–47.
42. Boyvat F, Harman A, Ozyer U, Aytekin C, Arat Z. Percutaneous sonographic guidance for TIPS in Budd-Chiari syndrome: direct simultaneous puncture of the portal vein and inferior vena cava. AJR Am J Roentgenol. 2008;191:560–4.