48uep6bbphidvals|398
48uep6bbph|2000F98CTab_Articles|Fulltext
We report the unusual occurrence of a patient with recurrent pancreatitis caused by the underlying presence of a benign mucinous cystadenoma of the distal pancreas. There are only two previous case reports of a mucinous cystadenoma causing acute pancreatitis[1,2] and two case reports of the coexistence of a pancreatic pseudocyst with a mucinous cystadenoma.[3,4]
Case Report
A 54-year old female was admitted for recurrent bouts of central abdominal pain radiating to back since last 6 months. Her past medical history was significant for hypertension, which was currently controlled. The admission laboratory tests showed normal liver function tests, normal white blood cell count, but her amylase and lipase were 216 U/L (normal range, 27-137 U/ L) and 2217 U/L (normal range, <190 U/L), respectively. She was treated for acute pancreatitis and was discharged 7 days later.
However, she returned six weeks after discharge with recurrent mid-epigastric pain with radiation to her back, nausea, and vomiting, and continued early satiety. Her laboratory tests again showed elevated amylase and lipase of 498U/L and 3025 U/L, respectively. Contrasted abdominal CT showed a 10 cm cystic mass in the midbody/tail of the pancreas with an anterior rim of normal appearing pancreas (Figure 1).
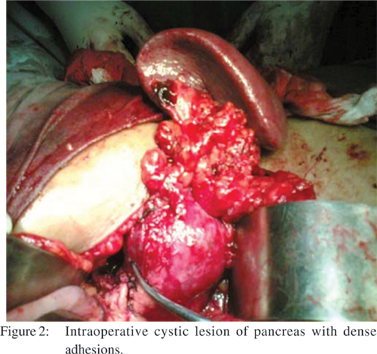
Endoscopic retrograde cholangiopancreatography (ERCP) revealed a normal caliber pancreatic duct with an abrupt cutoff at the distal duct. She underwent exploratory laparotomy. After gaining access to the lesser sac, a 10 cm cystic lesion was identified at the junction of the body and tail of the pancreas (Figure 2).
However, there were dense adhesions and inflammation surrounding the 10 cm cystic lesion at the junction of body and the tail of the pancreas. Enbloc distal pancreas and spleen was removed (Figure 3). The final pathology confirmed the presence of a mucinous cystadenoma with its characteristic features: a mucin-producing columnar epithelium lining the cyst wall, underlying ovarian-type stromal tissue, and no evidence of dysplasia. The patient had an uneventful recovery, began to tolerate oral intake, and was discharged 7 days after surgery.


Discussion
Mucinous
cystadenomas are a benign type of mucinous cystic neoplasm of the pancreas
(MCNs). Patients with mucinous cystadenomas have a mean presentation of 48
years with a distinct female predominance as high as 9:1 in some series.[5,6] These cystic tumors are typically large
(50% are greater than 5 cm) and most (greater than 50%) are located in the
distal body and tail of the pancreas. Patients most often present with nonspecific
abdominal pain, nausea, vomiting, and weight loss.
In
published series there are only two cases in the literature that attribute the
development of acute pancreatitis to a mucinous cystadenoma.[1,2] Likewise, there are only two cases that describe the coexistence
of a pseudocyst and a mucinous cystadenoma.[3,4]
There are no reported cases of a mucinous cystadenoma causing recurrent
pancreatitis. Rattner et al. found that in computed tomography neoplastic
process is highly suggestive by presence of solid components in the wall of the
cyst.[7] Most pseudocysts are single and have few solid
components, septae or loculations, or calcifications in the cyst wall. Hsieh
and colleagues reported that, magnetic resonance imaging easily differentiated
a pseudocyst from a mucinous cystadenoma.[4] T1-weighted,
T2-weighted, and gadolinium-enhanced images may delineate loculations, septae,
and further define the surrounding tissue in order to differentiate the cystic
lesions.
Endoscopic
ultrasound is a diagnostic tool which provides morphologic detail, and sampling
of fluid from the lesion for measurement of enzymes, viscosity, measurement of
tumor markers, and cytologic examination. Song et al. observed that pseudocysts
contained echogenic debris and demonstrated parenchymal changes more often than
cystic neoplasm. In addition, internal septae were seen with EUS more
frequently in cystic neoplasms.[8]
Cyst fluid
may be used for diagnosis by cytology and measurement of various tumor markers.
A high concentration of amylase in the cyst fluid is diagnostic of a pancreatic
psuedocyst.[9] Similarly, a high carcinoembryonic antigen
(CEA) is indicative of a mucinous cystadenoma and a CEA of greater than 400 is
potentially predictive of malignancy.[10] Mucin and mucinous
cells are characteristic of mucinous cystic neoplasm, glycogen-staining cells
are seen in serous cystadenomas, and inflammatory cells and histiocytes are
associated with seudocysts. Even pancreatic acinar cell carcinoma may present
with features of pancreatitis.[11] Fine needle aspiration cytology
provides tissue diagnosis in such cases.
Cystic pancreatic tumors constitute for about 10-15% of pancreatic cysts and less than 1% of pancreatic neoplasms.[12] World Health Organization (WHO) classified cystic pancreatic neoplasms into three main categories, mucinous variety (45%), serous variety (16%), and intraductal papillary mucinous neoplasm (IPMNs) (32%).[13] Sahani DV et al. have suggested a classification scheme for pancreatic cysts that is based on the imaging morphologic features of the cyst into four subtypes: (a) unilocular cysts, (b) microcystic lesions, (c) macrocystic lesions and (d) cysts with a solid component. Multiple unilocular cysts are most often pseudocysts resulting from prior pancreatitis.[14] Other causes of multiple cysts include von Hippel–Lindau disease and rarely IPMN. In von Hippel–Lindau disease, the pancreas is otherwise healthy and cysts may also be present in the kidneys or liver.[15] The presence of ovarian-type stroma has now becoming an essential requirement for the diagnosis of MCN, and when defined as such, MCN is seen almost exclusively in women of perimenopausal age group as thick-walled multilocular cystic mass in the tail of the pancreas.[16]
Benign and malignant cystic neoplasms are simply differentiated based on pathology. Any cystic neoplasm exhibiting invasive components is considered a malignant cystadenocarcinoma. For patients with benign serous or mucinous cystadenomas, complete resection is curative. Patients with resected mucinous cystadenocarcinomas typically live much longer than those with resected ductal adenocarcinoma, with an approximate 50 percent 5-year survival.[17]
References
1. Sperti C, Pasquali C, Davoli C, Polverosi R, Pedrazzoli S. Mucinous cystadenoma of the pancreas as a cause of acute pancreatitis. Hepatogastroenterology. 1998;45:2421–4.
2. Fischer CP, Pope I, Garden OJ. Mucinous cystic tumour of the pancreas presenting with acute pancreatitis. HPB (Oxford). 2001;3:271–3.
3. Russell RT, Sharp KW. Mucinous cystadenoma of the pancreas associated with acute pancreatitis and concurrent pancreatic pseudocyst. Am Surg. 2005;71:292–7.
4. Hsieh CH, Tseng JH, Huang SF. Co-existence of a huge pseudocyst and mucinous cystadenoma: report of a case and the value of magnetic resonance imaging for differential diagnosis. Eur J Gastroenterol Hepatol. 2002;14:191–4.
5. Sarr MG, Carpenter HA, Prabhakar LP, Orchard TF, Hughes S, van Heerden JA, et al. Clinical and pathologic correlation of 84 mucinous cystic neoplasms of the pancreas: can one reliably differentiate benign from malignant (or premalignant) neoplasms? Ann Surg. 2000;231:205–12.
6. Fernandez-del Castillo C, Targarona J, Thayer SP, Rattner DW, Brugge WR, Warshaw AL. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003;138:427–3; discussion 33–4.
7. Rattner DW, Fernandez-del Castillo C, Warshaw AL. Cystic pancreatic neoplasms. Ann Oncol. 1999;10 Suppl 4:104–6.
8. Song MH, Lee SS, Park JS, Seo DW, Lee SK, Kim MH, et al. Therole of endoscopic ultrasonography in pancreatic cystic lesions [abstract]. Gastrointest Endosc. 2002;55:AB249.
9. Brugge WR. Diagnosis and management of relapsing pancreatitis associated with cystic neoplasms of the pancreas. World J Gastroenterol. 2008;14:1038–43.
10. Van Dam J. EUS in cystic lesions of the pancreas. GastrointestEndosc. 2002;56:S91–3.
11. Thomas PC, Nash GF, Aldridge MC. Pancreatic acinar cell carcinoma presenting as acute pancreatitis. HPB (Oxford). 2003;5:111–3.
12. Fernandez-del Castillo C, Warshaw AL. Cystic neoplasms of the pancreas. Pancreatology. 2001;1:641–7.
13. Kloppel G, Luttges J. WHO-classification 2000: exocrine pancreatic tumors. Verh Dtsch Ges Pathol. 2001;85:219–28.
14. Sahani DV, Kadavigere R, Saokar A, Fernandez-del Castillo C, Brugge WR, Hahn PF. Cystic pancreatic lesions: a simple imaging-based classification system for guiding management. Radiographics. 2005;25:1471–84.
15. Friedrich CA. Von Hippel-Lindau syndrome. A pleomorphiccondition. Cancer. 1999;86:2478–82.
16. Murakami Y, Uemura K, Ohge H, Hayashidani Y, Sudo T, Sueda T. Intraductal papillary-mucinous neoplasms and mucinous cystic neoplasms of the pancreas differentiated by ovarian-type stroma. Surgery. 2006;140:448–53.
17. Wilentz RE, Albores-Saavedra J, Zahurak M, Talamini MA, Yeo CJ, Cameron JL, et al. Pathologic examination accurately predicts prognosis in mucinous cystic neoplasms of the pancreas. Am J Surg Pathol. 1999;23:1320–7.