48uep6bbphidvals|418
48uep6bbph|2000F98CTab_Articles|Fulltext
Introduction
Epidemiological studies reveal geographical associations between cassava consumption and the incidence of TCP in tropical countries such as India,[1] Nigeria,[2] Uganda,[3] Indonesia[4] and Brazil[5] but do not absolutely correlate with the endemic distribution of TCP.[6] Cassava, a drought tolerant tuber, grows on nutritionally poor soils, and is a source of large yield of starch.[7] Hence cassava was used as substitute to cereals like rice especially by the people of low-income strata. However cassava roots contain just 1.5% protein; and in particular are deficient in sulphur containing amino acids – methionine, and cysteine.[8] They are also deficient in vitamins and other nutrients.
The low nutritive quality and presence of cyanogenic glycosides (93% linamarin and 7% lotaustralin) has always been a source of concern.[9] Cyanide must be sequestered and metabolized to avoid inhibition of cytochrome c oxidase, mitochondrial electron transport and consequent energy failure. Approximately 80% of absorbed cyanide is metabolized to thiocyanate in the liver by the sulfur transferases (chiefly rhodanese) and is excreted in the urine.[10] The rate-limiting factor in this reaction is usually the availability of sulfur donors in the body (methionine and cysteine).[10] Therefore, the protein content in the diet is a critical element of this detoxification pathway. The processing and preparation of cassava may have modifying effects. A high carbohydrate and low protein diet was shown to cause pancreatic fibrosis in an experimental study,[11] raising the possibility that it is the nutritional composition of the diet that is important in the pancreatic injury.
In this study, we attempted to analyze serum rhodanese, thiocyanate, urinary inorganic sulfate / creatinine ratio, plasma methionine, cysteine, as well as blood antioxidant levels in TCP patients as well as and in healthy controls and compared the levels of those who consumed significant (>200 g/day) amounts of cassava with cassava non-consumers.
Methods
Tropical chronic pancreatitis was diagnosed by previously reported criteria.[12] Subjects who consumed cassava > 200 g/ day were included in the cassava consumers group and subjects who do not consume cassava were included in the cassava non-consumers group. Subjects who consume less than 200 g/day were excluded from the study.
Serum rhodanese was assayed by Sorbo method.[13] Serum thiocyanate was measured by the Butts method[14] following the WHO MONICA[15] project protocol. Urinary inorganic sulfate was measured by turbidimetry.[16] Urinary creatinine was determined by Jaffe’s alkaline picrate method.[17] Erythrocyte GSH content was estimated using the method described by Beutler et al.[18] Assay of glutathione peroxidase was carried out according to the method of Paglia and Valentine.[19] Erythrocyte SOD was measured as described by Winterbourn et al.20 Lipid peroxidation (TBARS) was quantified following the method of Jain et al.[21] Plasma vitamin C was measured by the method described by Okamura.[22] Hemoglobin was determined by cyanmethemoglobin conversion method.[23]
Differences in mean were calculated using one-way analysis of variance with Scheffe post hoc test. Nonparametric Mann- Whitney U test and Kruskal-Wallis test, as appropriate, were used to compare variables without a normal distribution. Pearson’s correlation was used for bivariate correlations. Biochemical values were expressed as the mean ± SE for comparison.
Results
The demographic characteristics of study population are given in Table 1.
There was a significant reduction in serum rhodanese activity in both cassava consumers and cassava non-consumer TCP patients as compared to controls. However, rhodanese activity was not significantly different between cassava consumer- and non-consumer TCP patients (Table 2).
Serum thiocyanate was significantly lower in cassava consumer TCP patients as compared to cassava consumer controls but not cassava non-consumer TCP patients (Table 2).Plasma methionine, cysteine and urinary inorganic sulfate / creatinine ratio was significantly lower in both cassava consumer and non-consumer TCP patients as compared to controls. However, plasma methionine, cysteine and urinary inorganic sulfate / creatinine ratio (an index of sulphur amino acid intake over long period) was comparable between cassava consumer and non-consumer TCP patients (Table 2).


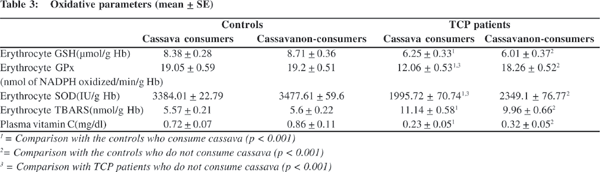
Among antioxidants, erythrocyte glutathione, glutathione peroxidase, superoxide dismutase and plasma vitamin C was significantly lower in TCP patients who consumed cassava and those who did not consume cassava as compared to controls. In addition, erythrocyte glutathione peroxidase and superoxide dismutase was significantly lower in TCP patients who consume cassava as compared to TCP patients who do not consume cassava (Table 3).
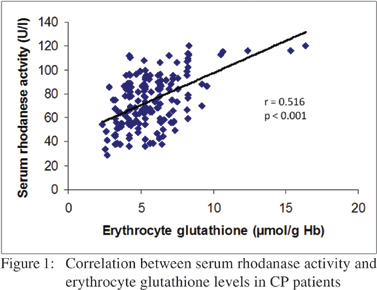
A significant positive correlation was noted (Figure 1) between erythrocyte glutathione and serum rhodanese activity (r = 0.516, p< 0.001).
Discussion
Initially studies suggested that chronic exposure to cyanides (e.g. by consumption of insufficiently processed cassava) was involved in etiopathogenesis of TCP.[24] A previous report showed that rats fed on a diet containing 22.8 g of cassava (containing 73 mg/g cyanide) and sacrificed at 18 months showed pancreatic changes such as dilated ductules, papillary infoldings, eosinophilic material in ductular lumen, round cell infiltration, and atrophic acini as is seen in TCP.[25] Okafor et al have reported elevation of serum amylase levels in rats fed cassava diet.[26] Sreeja and Leelamma[27] reported that in experimental animals given low protein and high cyanide, the amount of thiobarbituric acid reactive substances, conjugated dienes, and hydroperoxides was significantly high and also the activities of the antioxidant enzymes superoxide dismutase (SOD) and catalase were significantly low.
In this study, we demonstrated significant reduction in rhodanase activity in CP patients with concomitant decrease in sulfur amino acids. This finding would suggest an increased risk of defective detoxification of cyanide in these patients. We observed a positive correlation between erythrocyte glutathione and serum rhodanese activity. This would suggest that free radicals may suppress enzyme activity. Depletion in glutathione (GSH) levels to the extent observed in this study could further lead to drastic decrease in the total antioxidant status of the body. This is because glutathione (GSH) helps to recycle vitamins C and E (cellular antioxidants), blocks free radical damage, enhances the antioxidant activity of vitamin C, and plays a critical role in the detoxification of harmful compounds.[28]
Sandhyamani et al have demonstrated that feeding with a protein-deficient carbohydrate-rich diet induced vascular lesions in bonnet monkeys and resulted in pancreatic damage that resembles TCP.11 These changes were noticed irrespective of whether the source of the carbohydrate was cassava or corn starch. It is noteworthy that in patients with alcoholic pancreatitis (ACP) in France, Sarles observed that a high fat, high protein diet, and next, a very low fat diet also, predisposed his patients to alcoholic pancreatitis.[29] The senior author haspreviously reported a high carbohydrate, low protein and very low fat diet in a cohort of patients from south India with TCP.[30] It was suspected whether low fat content in these patients is responsible for susceptibility to pancreatitis.
Geevarghese and McMillan hypothesized that cassava (cyanogenic glycosides) consumption may play a role in development of tropical pancreatitis, but could not substantiate this in an animal experiment.6 But there is no evidence so far that cassava could be a sole etiological agent for pancreatitis. In fact, a case control study by Narendranathan et al[31] showed a lack of association with cassava consumption. Mathangi et al[32] did not find pancreatitis or diabetes in rats fed cassava even after 1 year of cassava consumption.
However, its role as a cofactor (due to its content of cyanogens, which is a tissue toxin), cannot be entirely ruled out. The fact that the poor and low middle class population of Kerala now eat lesser quantities of cassava than their predecessors and that the varieties that are cultivated and consumed now are less toxic ones could mean a lesser exposure to the cyanogen content than would have been the case earlier.[33] This perhaps might be reflecting the lack of significant differences in the cyanogenic detoxification markers and antioxidant status between the cassava consuming and nonconsuming TCP patients. Protein consumption by the Kerala population has also increased in the last couple of decades.
These facts might partially explain the later age of onset of the disease now. In addition, there are cofactors now that were not significant a couple of decades earlier. We have recently reported deficiencies in zinc and folate in CP patients.[34] Traditional strategies for reducing cyanogen toxicity in cassava foods aim at accelerating cyanogenesis and cyanide volatilization during food processing. However, it is often impossible to remove all the cyanogenic compounds through processing.[35] Recent reports of genetically modified cassava varieties rich in zinc, iron, protein, beta-carotene, and vitamin indicate novel ways by which cassava usage can be continued without health concerns in regions where it remains a staple food.[36] Another recent report describes proof of concept of how cassava was genetically modified to express zeolin, a nutritionally balanced storage protein; resulting in total protein levels of 12.5% dry weight within tissue (a four fold increase as compared to non-transgenic controls). This also resulted in nearly 55% reduction in cyanogenic content.[37]
In conclusion, a significant reduction in rhodanese activity with concomitant decrease in sulfur containing amino acids and antioxidants such as glutathione suggests that TCP patients are at higher risk of oxidative stress and probably of defective detoxification of cyanogens. However, there were no major differences between cassava consumers and nonconsumers.
This study does not prove a contributory effect of cassava to pancreatic damage in TCP. However, a contributory effect of food toxins, along with other dietary deficiencies such as low levels of sulphur amino acids in TCP cannot be totally negated.
Acknowledgements
We gratefully acknowledge the financial support from State Technology and Environment Council, Government of Kerala, India.
References
- Geevarghese PJ. Pancreatic Diabetes, Popular Prakasan, Bombay. 1968.
- Osuntokun BO. Cassava diet and cyanide metabolism in Wistar rats. Br J Nutr. 1970;24:797–800.
- Shaper AG. Aetiology of chronic pancreatic fibrosis with calcification seen in Uganda. Br Med J. 1964;1:1607–9.
- Zuidema PJ. Cirrhosis and disseminated calcification of the pancreas in patients with malnutrition. Trop Geogr Med. 1959;11:70–4.
- Dani R, Nogueira CE. Chronic calcifying pancreatitis in Brazil: analysis of 92 cases. Leber Magen Darm. 1976;6:272–5.
- McMillan DE, Geevarghese PJ. Dietary cyanide and tropical malnutrition diabetes. Diabetes Care. 1979;2:202–8.
- El-Sharkawy MA. Cassava biology and physiology. Plant Mol Biol. 2004;56:481–501.
- Montagnac JA, Davis CR, Tanumihardjo SA. Nutritional value of cassava for use as a staple food and recent advances for improvement. Comprehensive Reviews in Food Science and Food Safety. 2009;8:181–94.
- Nartey F. Studies on cassava, Manihot utilissima Pohl—I. Cyanogenesis: The biosynthesis of linamarin and lotaustralin in etiolated seedlings. Phytochemistry.1968;8:1307–12.
- World Health Organization. Hydrogen Cyanide and Cyanides: Human Health Aspects. Geneva, Switzerland: World Health Organization; 2004. Concise International Chemical Assessment Document 61.
- Sandhyamani S, Vijayakumari A, Balaraman Nair M. Bonnet monkey model for pancreatic changes in induced malnutrition. Pancreas. 1999;18:84–95.
- Balakrishnan V, Unnikrishnan AG, Thomas V, Choudhuri G, Veeraraju P, Singh SP, et al. Chronic pancreatitis. A rospective nationwide study of 1,086 subjects from India. JOP. 2008;9:593–600.
- Sorbo BH. Rhodanese. Methods Enzymol 1955;2:334–7.
- Butts WC, Kueheman M, Widdowson GM: Automated method for determining serum thiocyanate, to distinguish smokers fromnonsmokers. Clin Chem. 1974;20:1344–8.
- WHO MONICA Project, Monica Manual, Part III: Population Survey Sec 3: Standardization of thiocyanate measurements Nov 1990.
- Lundquist P, Mårtensson J, Sörbo B, Ohman S. Turbidimetry of inorganic sulfate, ester sulfate, and total sulphur in urine. Clin Chem. 1980;26:1178–81.
- Bonsnes RW, Taussky HH. Determination of creatinine in urine. In: Varley H, ed. Practical Clinical Biochemistry. 4th ed. London, UK: Heinemann; 1967.p.197–8.
- Beutler E, Duron O, Kelly BM. Improved method for the determination of glutathione. J Lab Clin Med. 1963;61:882–8.
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–69.
- Winterbourn CC, Hawkins RE, Brian M, Carrell RW. The estimation of red cell superoxide dismutase activity. J Lab Clin Med. 1975;85:337–41.
- Jain SK, McVie R, Duett J, Herbst JJ. Erythrocyte membrane lipid peroxidation and glycosylated hemoglobin in diabetes. Diabetes. 1989;38:1539–43.
- Okamura M. An improved method for determination of L ascorbic acid and L-dehydroascorbic acid in blood plasma. Clin Chem Acta. 1980;103:259–68.
- Drabkins DL, Austin JH. Spectrophotometric constants for common hemoglobin derivatives in human, dog and rabbit blood. J Biol Chem. 1932;98:719–3.
- Pitchumoni CS, Jain NK, Lowenfels AB, DiMagno EP, Chronic cyanide poisoning: unifying concept for alcoholic and tropical pancreatitis. Pancreas. 1988;3:220–2.
- Geevarghese PJ. Cassava diet, tropical calcifying pancreatitis and pa ncreatic diabetes. In: Delange F, and Ahluwalia R.(eds) Cassava Toxicity and Thyroid. Research and Public Health Issues. Ottawa, Canada: International Development Research Centre; 1983.p.77–8.
- Okafor PN, Anoruo K, Bonire AO, Maduagwu EN. The Role of Low-Protein and Cassava-Cyanide Intake in the Aetiology of Tropical Pancreatitis. Canadian J Pure and Applied Sciences 2008;2:393–7.
- Sreeja VG, Leelamma S. Effect of protein supplemented cassava diet in rats. Indian J Biochem Biophys. 1996;33:149–51.
- Meister A. Glutathione-ascorbic acid antioxidant system in animals. J Biol Chem. 1994;269:9397–400.
- Balakrishnan V, Lakshmi R, Nandakumar R. Tropical pancreatitis - what is happening to it? In: Balakrishnan V, Harish Kumar, Sudhindran S, Unnikrishnan AG (eds). Chronic Pancreatitis and Pancreatic Diabetes in India. Cochin: Indian Pancreatitis Study Group; 2006.p.23–54.
- Balakrishnan V, Sauniere JF, Hariharan M, Sarles H. Diet, pancreatic function, and chronic pancreatitis in south India and France. Pancreas. 1988;3:30–5.
- Narendranathan M, Cheriyan A. Lack of association between cassava consumption and tropical pancreatitis syndrome. J Gastroenterol Hepatol. 1994;9:282–5.
- Mathangi DC, Deepa R, Mohan V, Govindarajan M, Namasivayam A. Long-term ingestion of cassava (tapioca)
does not produce diabetes or pancreatitis in the rat model. Int J Pancreatol. 2000;27:203–8.
- Balakrishnan V, Nair P, Radhakrishnan L, Narayanan VA. Tropical pancreatitis - a distinct entity, or merely a type of chronic pancreatitis? Indian J Gastroenterol. 2006;25:74–81.
- Girish BN, Rajesh G, Vaidyanathan K, Balakrishnan V. Zinc status in chronic pancreatitis and its relationship with exocrine and endocrine insufficiency. JOP. 2009;10:651–6.
- Nambisan B, Sundaresan S. Effect of processing on the cyanoglucoside content of cassava. Journal of the Science of Food and Agriculture. 1985;36:1197–203.
- Nassar N, Ortiz R. Breeding cassava to feed the poor. Sci Am. 2010;302:78–82,84.
- Abhary M, Siritunga D, Stevens G, Taylor NJ, Fauquet CM. Transgenic biofortification of the starchy staple cassava (Manihot esculenta) generates a novel sink for protein. PLoS ONE 6(1):e16256.