48uep6bbphidvals|442
48uep6bbph|2000F98CTab_Articles|Fulltext
Hemobilia is a complex clinical problem and occurs when there
is an abnormal communication between the vascular and biliary
system. Although it was first described in 1654 by Francis
Glisson, it was not until 1948[1] that Sandblom gave a detailed
description of hemobilia. Profuse haemorrhage into the biliary
tract i.e. major hemobilia is rare but can lead to morbidity and
mortality. Although there is significant data available from the
west, there is little published information on this condition
from India. With the increasing number of invasive hepatobiliary
interventions being performed, the incidence of hemobilia is
likely to rise. The aim of this study was to analyze the spectrum,
clinical presentation and management of major hemobilia in a
tertiary referral centre from western India.
Methods
A retrospective analysis of 22 patients with major hemobilia
was undertaken over a 5-year period. There were 16 males and
6 females with a mean age of 39 years (range: 13-74 years).
Major hemobilia was defined as bleeding in the biliary tree
causing overt gastrointestinal bleeding in the form of
haematemesis/melena associated with a fall in haemoglobin of>3 gm/dl. Cholangitis was diagnosed when there was associated
fever and leukocytosis. All patients were resuscitated with
intravenous fluids and covered with broad spectrum antibiotics.
Upper gastrointestinal (UGI) endoscopy was performed in case
of doubt about the source of bleed.
Results
The main presenting symptoms were melena in 20 patients and
hemetemesis (with melena) in 8 patients. Seventeen patients
needed blood transfusions (mean: 3 units, range: 1-5 units).
The mean drop in hemoglobin was 5 gm/dl (range: 3–7 gm/dl).
Eight patients presented with associated cholangitis and 3 of
these developed septic shock.Thirteen patients (59%)
developed hemobilia secondary to iatrogenic causes
(percutaneous transhepatic biliary drainage 8, post
laparoscopic cholecystectomy 3, endoscopic retrograde
cholangiopancreatography 1, and liver biopsy 1). There was
history of trauma in 6 patients and 3 patients bled from liver
tumors. The hemobilia was seen immediately in the endoscopic
papillotomy patient, but developed after a mean of 6 days in
the post percutaneous transhepatic biliary drainage (PTBD)
group and 11 days in the trauma group. Four out of the six
patients with trauma had undergone laparotomy elsewhere,
with 3 requiring suturing of liver lacerations.
Eight patients had an UGI endoscopy and fresh blood was
seen in the second part of duodenum in five. Ultrasound
examination with Doppler was performed in 10 patients, which
revealed filling defects in the biliary tree suggestive of clots (3
patients), pseudoaneurysm (6 patients) and was normal in one
patient. Doppler was performed in some cases elsewhere before
referral.
Abdominal angiography (celiac & SMA) was performed in
20 out of the 22 patients. Angiography was not done in two
patients (one patient had venous bled from portal biliopathy
after stone extraction and the other underwent surgery directly
for liver tumor, since the bleeding had stopped). Angiography
revealed pseudoaneurysm of the right hepatic artery or its
branches in 14 patients, left hepatic artery in 2, an arterio-biliary
fistula in 1, tumor blush in 1 and the source could not be located
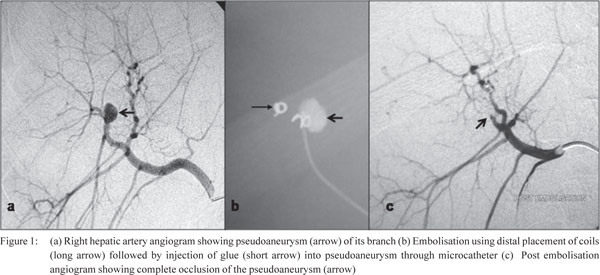
in 2 patients. Figure 1 (a, b, c) shows traumatic pseudoaneurysm
of a branch of right hepatic artery along with its embolisation.
Seventeen patients were treated with radiological intervention
in the form of embolisation (coils and/or glue-16, and
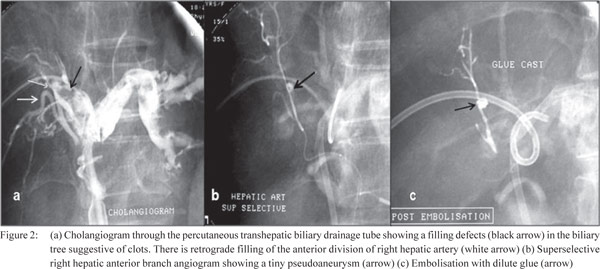
chemoembolisation with doxorubicin –1). Figure 2 (a, b, c)
shows a PTBD induced psuedoaneurysm with
angioembolisation. Sixteen patients responded immediately to
embolisation. One patient required two sessions, as he had an
accessory right hepatic artery arising from the superior
mesenteric artery which was the cause of bleed and was missed
on the initial angiogram. Three patients required surgery (liver
resection for hepatocellular carcinoma – 2, laparotomy for
bleeding from a portal biliopathy – 1) and two were managed
conservatively. The cholangitis did not need separate drainage
but settled with control of the hemobilia.
There were two early deaths (mortality: 9%). One patient
with portal cavernoma died of massive venous bleed, which
could not be controlled at laparotomy and the other due to
liver failure 1 week after hemoembolisation. The other two
patients with liver tumors died during the follow up due to
extensive disease. Out of the eight patients who had undergone
PTBD for hilar cholangiocarcinoma (4 preoperative biliary
drainage, 4 palliative drainage and stenting), 4 were lost to
follow up and 4 underwent definitive surgery once the bilirubin
was < 3 mg/dl. Two patients in the post cholecystectomy group
underwent definitive biliary repair after 3 months. Table 1
summarizes the etiology, angiography findings, management
and outcome of our patients.
Discussion
In the first large review of hemobilia consisting of 545 patients
published by Sandblom in 1972,[2] trauma was noted as the most
common causative factor. In developed countries the increasing
use of interventional procedures has now resulted in iatrogenic
injury in being the most common cause.[3]
The incidence of iatrogenic hemobilia following liver biopsy,
percutaneous transhepatic cholangiography and PTBD is 1%[4]
and 2-10%[5,6] respectively. The commonest iatrogenic cause of
hemobilia in our series was secondary to PTBD procedures,
which was seen after mean of 6 days. A combination of pressure
from a stiff catheter and local sepsis causes erosion of the
blood vessel wall, leading to formation of a pseudoaneurysm in close proximity to the bile duct, causing hemobilia on rupture.
The incidence of blunt liver injury causing hemobilia is
about 2.5 - 3%.[7] Out of the 6 patients with liver trauma in our
series, 3 had undergone hepatorrhaphy elsewhere. The frequent
reason is bleeding within the liver substance with intact capsule
or superficial suturing of the injury, with resultant hematoma
rupturing into the biliary tree.[8] The hematoma may also get
infected leading to a mycotic pseudoaneurysm, which may
later erode into the biliary tree. Therefore, while exploring a
liver laceration, precise identification and suturing of vessels
and ducts is important. The trend towards conservative
management of liver injury may be expected to result in an
increase in the incidence of hemobilia.[9] Therefore we believe
that there should be a low threshold for follow up imaging in
this group of patients.
Tumors account for about 6% of all causes of major hemobilia
and malignant tumors outnumber the benign ones.[10,11]
Hepatocellular carcinoma secondary to cirrhosis is the
commonest tumor causing hemobilia.[12] The three tumor
patients in our series represent an interesting group and all of
them had small lesions.
The classic triad of upper abdominal pain, GI bleed and
jaundice,[13] was seen only in a minority of our patients. We
believe that for any patient undergoing hepatobiliary
intervention and subsequently presenting with persistent
abdominal pain with pallor, there should be a low thereshold in
suspecting hemobilia. Side viewing endoscopy can identify
bleeding from the papilla in only 30% of patients.[14] We could
demonstrate fresh blood in the second part of duodenum on
UGI scopy in 62% (5/8 patients). Pseudoaneurysms are noted
as well circumscribed anechoic lesions on ultrasound and
doppler shows a turbulent flow within,[15] as was seen in 60% (6/
10 patients) of our patients. CECT can demonstrate smaller
hematomas, anatomical variations, pseudoaneurysms and
cavitating lesions.[16]
Once hemobilia is strongly suspected, the most useful study
is angiography, which may reveal the precise source of bleed.[17]
It can also be combined with definitive therapy by radiological
intervention. Celiac axis angiography should always be
accompanied with superior mesenteric arteriography because
anomalous/accessory right hepatic artery may originate from
the SMA in almost 20 % of the patients, and was observed in one of our patients. Selective right and left hepatic angiogram
may be performed especially in cases of hemobilia secondary
to PTBD, as nonselective angiograms may miss small
pseudoaneurysms. The angiogram may sometimes appear
normal in the absence of any active bleeding or when there is
no demonstrable lesion. Hence we feel that if bleeding
continues or recurs it may be worth repeating an angiogram.
Angioembolisation has now become the first line of
treatment and involves selective occlusion with permanent
embolic agents like microcoils and cyanoacrylate glue.[18] Coils
induce thrombosis, hence with gross coagulopathy, the vessel
may remain patent. Glue can be used to treat smaller
pseudoaneurysm where coil placement may be difficult. Also it
conforms to the shape of pseudoaneurysm and forms a cast
instantly, even in the presence of coagulopathy, and is much
cheaper as compared to coils. Combination of coil and glue
also can be used.[19] Ideally embolisation distal and proximal to
the pseudoaneurysm is necessary to prevent collateral filling
of the pseudoaneurysm. Alternatively, complete occlusion of
the pseudoaneurysm with coils or glue may be done followed
by proximal occlusion with coils.
Previous reviews and retrospective series have shown the
success rate of transarterial embolisation (TAE) to be in the
range of 80 – 100%.4 We had a success rate of 90%. Failure may
be due to technical reasons or extensive collaterals. Antibiotic
prophylaxis is recommended.[20] Selective embolisation as close
to the pseudoaneurysm or fistula possible is desirable to reduce
the likelihood of both recurrence and hepatic necrosis.
Emergency surgery to control major hemobilia is difficult
and should be avoided, as the results are poor. It may be better
to transfer the patient to a center with angiography facilities
rather than operate. Operative intervention may be required if
radiological expertise is not available, there is failure of TAE, or
manifestation of hepatic sepsis.[21] It involves ligation of the
bleeding vessel and hepatic resections. If bleeding is not
controlled by ligation or there is severe trauma an urgent liver
resection may have to be performed. Our mortality of 9% was
slightly higher than that the 5% reported in literature.[3]
There is paucity of literature regarding the etiology,
management and outcome of hemobilia from India. The
published case reports from India mainly describe hemobilia
following trauma or cholecystectomy.[22,23] In the only published
series by Srivastava et al,[24] the predominant etiology of
hemobilia was liver injury following road traffic accidents.
It highlighted the role of TAE in the management of hemobilia.[24] Our series highlights the changing spectrum of hemobilia from
liver injuries to hepatobiliary interventions in our country,
mirroring the spectrum of the developed world. This could be
due to a referral bias. It also emphasizes the role of angiography
in its diagnosis and management. A high index of suspicion
and timely intervention is important. In India the increasing
incidence of biliary stone disease, as well as increasing
nterventions on the hepatobiliary system are likely to result in
clinicians encountering this problem more frequently.
References
- Sandblom P. Hemorrhage into the biliary tract following trauma; traumatic hemobilia. Surgery. 1948;24:571–86.
- Sandblom P. Hemobilia. Surg Clin Nor Am. 1973;53:1191–201.
- Green MH, Duell RM, Johnson CD, Jamieson NV. Hemobilia. Br J Surg. 2001;88:773–86.
- Yoshida J, Donahue PE, Nyhus LM. Hemobilia: a review of recent experience with a world wide problem. Am J Gastroenterol. 1987;82:448–53.
- Dousset B, Sauvanet A, Bardou M, LegMan P, Vilgrain V, Belghiti J. Selective surgical indications for iatrogenic hemobilia. Surgery. 1997;121:37–41.
- Jeng KS, Ohta I, Yang FS. Reappraisal of the systematic management of complicated hepatolithiasis with bilateral intrahepatic biliary strictures. Arch Surg. 1996;131:141–7.
- Parks RW, Chrysos E, Diamond T. Management of liver trauma. Br J Surg. 1999;86:1121–35.
- Merrell SW, Schneider PD. Hemobilia—evolution of current diagnosis and treatment. West J Med. 1991;155:621–5.
- Carrillo EH, Platz A, Miller FB, Richandran JD, Polk HC Jr.
Non-operative management of blunt hepatic trauma. Br J Surg. 1998;85:461–8.
- Blumgart LH, Fong Y. Surgery of the liver and biliary tracts. W.B. Saunders Company Ltd; 2000.p.1319–39.
- John A, Ramachandran TM, Ashraf S, Nair MS, Devi RS. Carcinoma gall bladder presenting as hemobilia. Indian J Gastroenterol. 1999;l18:88–9.
- Sabiston DC. Textbook of surgery. 6th ed. W.B. Saunders Company Ltd; 2001.p.1056–9.
- Quincke H. Ein fall von aneurysm der leberarterie. Klin Wochenschr. 1871;8:349–51.
- Curet P, Baumer R, Roche A, Grellet J, Mercadier M. Hepatic
hemobilia of traumatic or iatrogenic origin: recent advances in diagnosis and therapy, review of the literature from 1976 to 1981. World J Surg. 1984;8:2–8.
- Sax SL, Athey PA, Lamki N, Cadavid GA. Sonographic finding in traumatic hemobilia: report of two cases and review of the literature. J Clin Ultrasound. 1988;16:29–34.
- Yokota J, Sugimoto T. Clinical significance of peirportal tracking on computed tomographic scan in patients with blunt liver trauma. Am J Surg. 1994;168:247–50.
- Shapiro MJ. The role of the radiologist in the management of gastrointestinal bleeding. Gasroenterol Clin North Am. 1994;23:123–81.
- Wallace S, Giaturco C Anderson JH, GoldsteiN HM, Davis
LJ, Bree RL. Therapeutic vascular occlusion utilizing steel coil technique: clinical applications. AJR Am J Roentgenol. 1976;127:381–7.
- Yamakado K, Nakatsuka A, Tanaka N, Takano K, Matsumura K, Takeda K. Transcatheter arterial embolisation of ruptured
pseudoaneursym with coil and n-butly cyanoacrylate. J Vasc Interv Radiol. 2000;11:66–72.
- Cho KJ, Reuter SR, Schmidt R. Effect of experimental hepatic artery embolisation on hepatic function. Am J Roentgenol. 1976;127:563–7.
- Moodley J, Singh B, Lalloo S, Pershad S, Robbs JV. Non-operative management of hemobilia. Br J Surg. 2001;88:1073–6.
- Bajpai M, Bhatnagar V, Mitra DK, Upadhyaya P. Surgical
management of traumatic hemobilia in children by direct ligation
of the bleeding vessel. J Pediatr Surg. 1989;24:436–7.
- Moses V, Keshava SN, Wann VC, Joseph P, Sitaram V. Cystic
artery pseudoaneurysm after laparoscopic cholecystectomy presenting as haemobilia: a case report. Trop Gastroenterol. 2008;29:107–9.
- Srivastava DN, Sharma S, Pal S, Thulkar S, Seith A, Bandhu S, et
al. Transcatheter arterial embolisation in the management of hemobilia. Abdom Imaging. 2006:31:439–48.