Mannina EM, Kandil E
Department of Endocrine Surgery,
Tulane University Health Sciences Center,
1430 Tulane Ave., SL-22
New Orleans, LA 70112, USA
Corresponding Author:
Dr. Mannina
Email: emannina@tulane.edu
48uep6bbphidvals|502 48uep6bbph|2000F98CTab_Articles|Fulltext With an incidence between 1-4 cases per million population, insulinomas are very rare, though they are the most common functional neuroendocrine neoplasm of pancreatic origin.1 Tumor localization with calcium gluconate arterial stimulation with hepatic venous sampling (ASVS) for insulin has been shown in cases of unsatisfactory imaging or suspected tumors smaller than 1cm. The surgeon’s goal is to cure the patient with minimal morbidity, and confidence in localization of the lesion is a means to this end. This case contributes to the literature by demonstrating the indispensable utility of calcium gluconate arterial stimulation with hepatic venous sampling in the definitive localization of a pancreatic insulinoma.
Case report
A 54-year-old female presented with recent history of frequent episodes of feeling sweaty, panicky, anxious and shaky. She reported waking up in the night with these symptoms and eating often provided relief. She denied loss of consciousness, confusion or recent hospital admissions. A review of symptoms
was unremarkable save an unintentional 10 pound weight gain. Her family history was significant for exocrine pancreatic cancer in her father. Vital signs and physical exam were within normal limits.
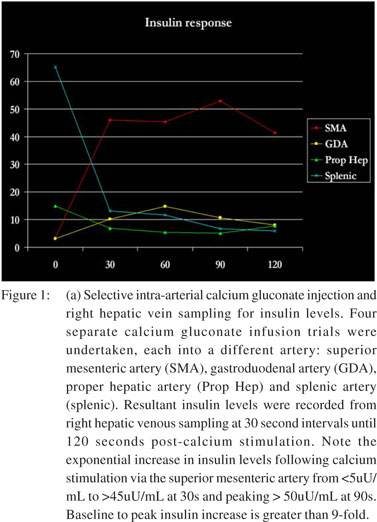
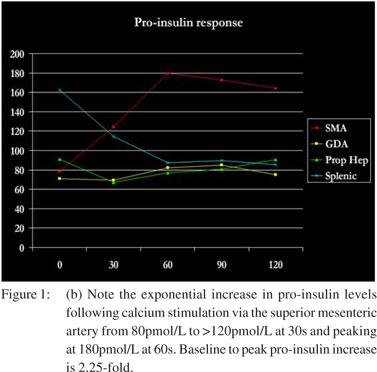
Suspecting hypoglycemia, her workup revealed a random plasma glucose of 38 mg/dL, serum insulin of 4.8 µU/mL (normal: 2.6-24.4 µU/mL) and serum pro-insulin of 75.6 pmol/L (normal: <18.8 pmol/L). Notably, serum sulfonylurea levels were not detected. A fasting glucose tolerance test demonstrated the following 9:00 am values: serum glucose = 61 mg/dL, insulin = 7.4 µU/mL, pro-insulin = 58.3 pmol/L and C-peptide = 2.4 ng/ mL (normal: 0.8-3.1 ng/mL). The 11:00 pm values were serum glucose = 50 mg/dL, insulin = 4.2 µU/mL, pro-insulin = 59.4 pmol/L and C-peptide = 2.7 ng/mL. Endoscopic ultrasound (EUS) revealed a poorly-defined hypo-echoic lesion in the pancreatic head approximately 10x9 mm in size FNA was non-diagnostic. CT scan with contrast poorly supported the EUS findings with minimal enhancement . To further localize the lesion, pre-operative hepatic venous sampling of insulin (Figure 1a) and pro-insulin (Figure 1b) levels at 30 second intervals following selective intra-arterial calcium gluconate injection into the superior mesenteric, gastroduodenal, hepatic and splenic arteries was performed. No rise in insulin or pro-insulin was observed with intra-arterial injection of calcium gluconate into the gastroduodenal artery, hepatic artery or splenic artery. Calcium gluconate injection into the superior mesenteric artery, however, produced a precipitous rise in hepatic venous insulin to a sustained value >40 µU/mL and hepatic venous pro-insulin to a sustained value >160 pmol/L. This data further localized the suspected insulinoma to the head of the pancreas.
 Enucleation of the suspected lesion was performed and a secretin stimulation test revealed no leakage from the fibrin glue sealed pancreas. Immediate post surgical serum glucose was 91 mg/dL compared to 51 mg/dL pre-operatively. Surgical pathology confirmed a 7 mm well-differentiated neuroendocrine neoplasm that stained positive for insulin and negative for glucagon. As of last follow-up, the patient was normoglycemic and asymptomatic.
Discussion
Nearly 90-95% of insulinomas are benign adenomas differentiated most reliably from their malignant counterparts by lack of metastasis.1 90% of tumors are less than 2 cm in size with 30% less than 1 cm resulting in few steric symptoms. Whipple established a triad of concerning symptoms: vision changes, amnesia, fatigue and possibly seizures, coma, or death resulting from <45 mg/dL glucose delivery to the CNS (“neuroglycopenia”); anxiousness, tremor, sweating from blood glucose levels <50 mg/dL (“hypoglycemia”); and relief of symptoms with administration of glucose (“relief with feeding”).[1] Typical workup involves a 72 hour fasting trial where periodic serum measurements of blood glucose, pro-insulin precursor, insulin and C-peptide are taken. Millan et al explored the localization of insulinomas via pancreatic venous sampling for insulin levels in the 1970’s.[2] In the early 1990’s investigators added an arterial infusion of calcium, a known promoter of insulin secretion by b cells, to assist in detecting insulinomas smaller than 15mm[3]. A study found intra-arterial calcium infusion with right hepatic vein insulin sampling to have a sensitivity for detecting insulinoma of 88% compared to 9% for ultrasound, 17% for CT scan, 43% for MRI, 36% for arteriography and 67% for portal venous sampling;[4] these findings have been replicated.[5-7] The protocol was modified by the use of low dose calcium gluconate, sampling of the right hepatic vein only and optional inclusion of the distal splenic artery for calcium stimulation.[5]
Enucleation of the suspected lesion was performed and a secretin stimulation test revealed no leakage from the fibrin glue sealed pancreas. Immediate post surgical serum glucose was 91 mg/dL compared to 51 mg/dL pre-operatively. Surgical pathology confirmed a 7 mm well-differentiated neuroendocrine neoplasm that stained positive for insulin and negative for glucagon. As of last follow-up, the patient was normoglycemic and asymptomatic.
Discussion
Nearly 90-95% of insulinomas are benign adenomas differentiated most reliably from their malignant counterparts by lack of metastasis.1 90% of tumors are less than 2 cm in size with 30% less than 1 cm resulting in few steric symptoms. Whipple established a triad of concerning symptoms: vision changes, amnesia, fatigue and possibly seizures, coma, or death resulting from <45 mg/dL glucose delivery to the CNS (“neuroglycopenia”); anxiousness, tremor, sweating from blood glucose levels <50 mg/dL (“hypoglycemia”); and relief of symptoms with administration of glucose (“relief with feeding”).[1] Typical workup involves a 72 hour fasting trial where periodic serum measurements of blood glucose, pro-insulin precursor, insulin and C-peptide are taken. Millan et al explored the localization of insulinomas via pancreatic venous sampling for insulin levels in the 1970’s.[2] In the early 1990’s investigators added an arterial infusion of calcium, a known promoter of insulin secretion by b cells, to assist in detecting insulinomas smaller than 15mm[3]. A study found intra-arterial calcium infusion with right hepatic vein insulin sampling to have a sensitivity for detecting insulinoma of 88% compared to 9% for ultrasound, 17% for CT scan, 43% for MRI, 36% for arteriography and 67% for portal venous sampling;[4] these findings have been replicated.[5-7] The protocol was modified by the use of low dose calcium gluconate, sampling of the right hepatic vein only and optional inclusion of the distal splenic artery for calcium stimulation.[5]
Interpretations of hepatic vein insulin levels were established: 4X increase (injected artery is direct tumor supply), 2-4X increase (injected artery is collateral tumor supply) and <2X increase (normal physiology).[5] Arteries chosen for calcium gluconate injection and their respective vascular territories included gastroduodenal artery (head of pancreas), superior mesenteric artery (head of pancreas), proper hepatic artery (liver) and splenic artery (body and tail of pancreas).[8]
Arterial stimulation with hepatic venous sampling (ASVS) was a more sensitive localizing modality than intra-operative ultrasound and palpation which yielded a sensitivity of only 77% compared to 92% for ASVS.7 In patients whose source of hyperinsulinemia could not be detected by conventional imaging, ASVS detected 76.9% of insulinomas confirmed by surgical pathology.[9]
Though studies outside the U.S. continued to recommend against ASVS to reduce costs,[10,11] a place for ASVS was established via its utility in detecting insulinomas with atypical/ poor imaging findings[12] as in our case, recurrent lesions[13] and operative-occult lesions.[14] To settle the discordance, a study by the National Institute of Diabetes and Digestive and Kidney Disease at the NIH found that ASVS was “vastly superior” to abdominal ultrasound, CT or MRI as a pre-operative test for localizing insulinomas.[15] Pre-operative ASVS for fine localization of insulinomas is critical for enucleation, to avoid partial pancreatectomy and its long term sequelae[16] and to prevent closing up a patient mid-operation due to failed intraoperative identification of the tumor.[14]
References
- Vaidakis D, Karoubalis J, Pappa T, Piaditis G, Zografos GN. Pancreatic Insulinoma: current issues and trends. Hepatobiliary Pancreat Dis Int. 2010;9:234–41.
- Millan VG, Urosa CL, Molitch ME, Miller H, Jackson IM.Localization of occultinsulinoma by super selective pancreatic venous sampling for insulin assay through percutaneous transhepatic catheterization. Diabetes. 1979;28:249–51.
- Doppman JL, Miller DL, Chang R, Shawker TH, Gorden P, Norton JA. Insulinomas: localization with selective intraarterial injection of calcium. Radiology. 1991;178:237–41.
- Doppman JL, Chang R, Fraker DL, Norton JA, Alexander HR, Miller DL, et al. Localization of insulinomas to regions of the pancreas by intra-arterial stimulation with calcium. Ann Intern Med. 1995;123:269–73.
- Defreyne L, König K, Lerch MM, Hesse UJ, Rottiers R, Feifel G, et al. Modified intra-arterial calcium stimulation with venous sampling test for preoperative localization of insulinomas. Abdom Imaging. 1998;23:322–31.
- Lo CY, Chan FL, Tam SC, Cheng PW, Fan ST, Lam KS. Value of intra-arterial calcium stimulated venous sampling for regionalization of pancreatic insulinomas. Surgery. 2000;128:903–9.
- Kirchhoff TD, Merkesdal S, Frericks B, Brabant G, Scheumann G, Galanski M, et al.Intraarterial calcium stimulation (ASVS) for pancreatic insulinoma: comparison of preoperative localization procedures. Radiologe. 2003;43:301–5.
- Jackson JE. Angiography and arterial stimulation venous sampling in the localization of pancreatic neuroendocrine tumours.Best Practice and Research Clinical Endocrinology & Metabolism. 2005;19:229–39.
- Moreno Moreno P, Gutiérrez Alcántara C, Muñoz-Villanueva MD, Ortega RP, Corpas Jiménez MD, Zurera Tendero L, et al.Usefulness of arterial calcium stimulation with hepatic venous sampling in the localization diagnosis of endogenous hyperinsulinism. Endocrinol Nutr. 2010;57:95–9.
- Chung JC, Choi SH, Jo SH, Heo JS, Choi DW, Kim YI.Localization and surgical treatment of the pancreatic insulinomas.ANZ J Surg. 2006;76:1051–5.
- Wong M, Isa SH, Zahiah M, Azmi KN.Intraoperative ultrasound with palpation is still superior to intra-arterial calcium stimulation test in localisinginsulinoma. World J Surg. 2007;31:586–92.
- Morita S, Machida H, Kuwatsuru R, Saito N, Suzuki K, Iihara M, et al.Preoperative localization of pancreatic insulinoma by super selective arterial stimulation with venous sampling. AbdomImaging. 2007;32:126–8.
- Ravi K, Britton BJ.Surgical approach to insulinomas: are preoperative localisation tests necessary? Ann R Coll Surg Engl. 2007;89:212–7.
- Rostambeigi N, Thompson GB.What should be done in an operating room when an insulinoma cannot be found? Clin Endocrinol (Oxf). 2009;70:512–5.
- Guettier JM, Kam A, Chang R, Skarulis MC, Cochran C, Alexander HR, et al. Localization of insulinomas to regions of the pancreas by intraarterial calcium stimulation: the NIH experience. J Clin Endocrinol Metab. 2009;94:1074–80.
- Goh BK, Ooi LL, Cheow PC, Tan YM, Ong HS, Chung YF, et al. Accurate preoperative localization of insulinomas avoids the need for blind resection and reoperation: analysis of a single institution experience with surgically treated tumors over 19 years. J Gastrointest Surg. 2009;13:1071–7.
|