48uep6bbphidvals|370
48uep6bbph|2000F98CTab_Articles|Fulltext
Introduction
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease of unknown etiology characterized by progressive strictures of the intra- and extrahepatic biliary ducts, leading to end-stage liver disease unless liver transplantation is performed.[1] Currently there are no effective therapies available to prevent or reverse disease progression. The development of cholangiocarcinoma (CCA) is observed in 8% to 36% of PSC patients further worsening overall prognosis and leading to ineligibility for liver transplantation.[2]
CCA is an insidiously progressive, malignant epithelial tumor arising from the biliary tree often following chronic biliary inflammation.[3] CCA is difficult to diagnose in patients with or without PSC, as classic tumor markers (CA 19-9, CEA, CA 125) exhibit poor sensitivity and specificity. Newer tumor markers such as Tu M2-Pk, matrix metalloproteinases, and KL-6 may provide future diagnostic support, but currently warrant additional investigation.[4,5,6] Furthermore, brush cytology obtained by percutaneous transhepatic cholangiogram (PTC) or endoscopic retrograde cholangiogram (ERC) has a very low diagnostic yield. Both modalities also carry risk for provoking bacterial cholangitis. Magnetic resonance cholangiopancreatography (MRCP) or endoscopic ultrasonography (EUS) with fine needle aspiration (FNA) are now preferred modalities of diagnosis and staging of CCA.
We report a case of de novo cholangiocarcinoma following re-transplantation of a patient with recurrent primary sclerosing cholangitis (rPSC) 20 years following orthotopic liver transplantation (OLT).
Case Report
A 40 year-old Jamaican male presented to our institution for evaluation of repeat OLT following rPSC in his liver allograft with notable hepatocellular decompensation. The patient underwent initial liver transplantation at another institution in 1988. The explanted liver had no pathologic evidence of CCA. Following OLT, the patient was diagnosed with ulcerative colitis (UC) and received treatment with 5-aminosalicylic acid (5-ASA) compounds. The remainder of his post-operative course was unremarkable until August 2008. In August 2008 he developed evidence of common hepatic duct/anastomotic biliary stricture with resultant intrahepatic biliary dilation and persistent cholangitis.
During his recurrent hospitalizations from August 2008 to January 2009, he was found to have arterial-portal venous shunting requiring embolization of a right hepatic arterial branch vessel. Computed tomography (CT) of the abdomen and pelvis and abdominal ultrasound (US) showed two 1-2cm indeterminate lesions noted in the 6th and 7th hepatic segments. These lesions were considered either tumor or bilomas based on the imaging findings. A biopsy of the background liver was performed in January 2009. Duct loss was observed in only a minority of the portal tracts (4 out of 19). Given the minimal degree of duct loss combined with evidence of cholestasis, portal fibrosis, bile duct injury, and previous clinical history of PSC, the pathologic review was most consistent with rPSC. Prior to transfer from the outside hospital, blood cultures were negative and his fever had subsided.
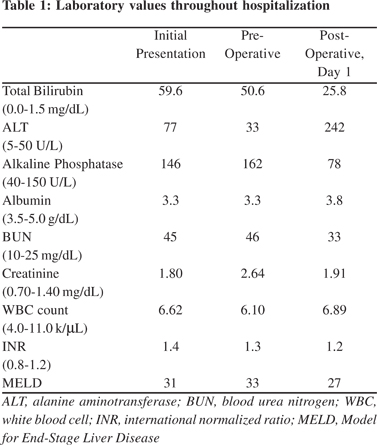
The patient presented to our facility in January 2009. His laboratory values on presentation are noted in (Table 1). On admission, his medications included tacrolimus, prednisone, ursodeoxycholic acid, and balsalazide. His tacrolimus level was within normal limits, remote hepatitis panel was negative, and cytomegalovirus (CMV) DNA testing was negative for evidence of CMV viremia.
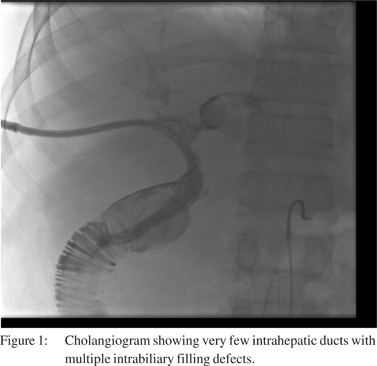
Shortly after admission he developed an E. coli bacteremiawhich was managed with intravenous antibiotic therapy and recurrent exchange of his biliary catheter. Repeat cholangio grams (Figure 1) demonstrated mild intrahepatic dilatation with multiple intrabiliary filling defects thought to be blood clots as evidence of active bleeding was observed in his biliary catheter. The patient had multiple episodes of gastrointestinal bleeding requiring significant blood transfusion and intensive care monitoring. Localization of the bleed was unsuccessful using cholangiography, angiography, and endoscopy. Due to persistent active bleeding despite an adequate platelet count and controlled international normalized ratio (INR), he was evaluated for an underlying coagulopathy.
Extensive evaluation revealed a poorly defined bleeding disorder related to low levels of Factor XI and Factor XII that was controlled with transfusions of fresh frozen plasma and intermittent dosing of recombinant coagulation factor VIIa, along with packed red blood cells as needed.
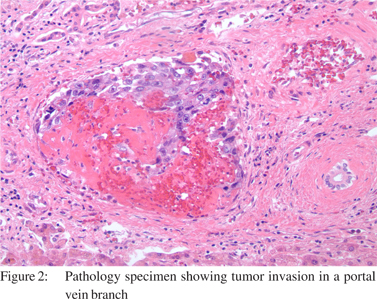
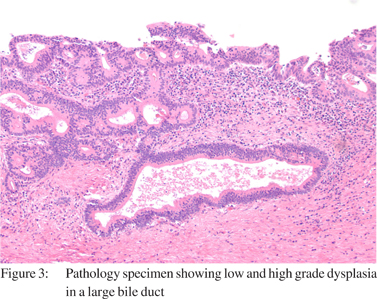
The patient was cleared for surgery and listed with a Model for End Stage Liver Disease (MELD) score of 30. The patient successfully underwent repeat OLT with a cadaveric organ and Roux-en-Y reconstruction. Following transplantation, he had an uneventful peri-operative course. Upon histologic evaluation, the explanted liver was found to have multifocal intrahepatic CCA (Figure 2 and 3) involving both lobes of the liver and the common hepatic duct, with 20% tumor necrosis and extensive angiolymphatic space invasion. The vascular margins were free of tumor. Postoperative carbohydrate antigen 19-9 (CA 19-9) was mildly elevated at 40 U/mL (0-33 U/mL). Laboratory values in the immediate post operative period are noted in (Table 1). Initially, the patient had an uneventful post operative course. Three months following his second OLT, evidence of recurrent CCA was identified in his new liver allograft. He died 3 weeks later due to complications of metastatic CCA.
Discussion
We present a case of de novo cholangiocarcinoma arising in the explanted liver of a patient who underwent repeat OLT for rPSC occurring 20 years after his initial transplant. No evidence of CCA was noted prior to his first transplant or in his initial liver explant. Only two similar such cases have been described in the literature.[7,8]
The overall risk for development of CCA in patients with PSC varies from 8% to 36% and CCA is frequently multifocal in this population.[2] The incidence of de novo CCA developing in an allograft is much lower. Resection and/or liver transplantation are the only curative options for patients with CCA. CCA constitutes an absolute contraindication to OLT at our facility due to the frequent disease relapse and high mortality. In centers which perform OLT for CCA, the 5-year survival rate following resection is 20% to 40% with a 10% intraoperative mortality.[9] In patients who undergo OLT for PSC and CCA, outcomes are even worse with 5-year post-operative survival as low as 10%.[10,11] Studies have shown that patients with incidental CCA at the time of OLT have improved early survival when compared to those patients transplanted for CCA, but intermediate- and long-term rates were similar.[12,13,14] This clinical course is consistent with that of our patient, as he did well initially following surgery but was found to have recurrent CCA three months post-transplantation. PSC remains the most common risk factor for the development of CCA in Western countries, but any chronic inflammatory biliary tract disease can be implicated.[10,15]
The risk of rPSC in patients 5 years post OLT is 12%, and 20% at 10 years.[16] Several recent single center studies have observed multiple risk factors predictive of disease recurrence following OLT. Evidence of CCA prior to OLT,[16] presence of UC post- OLT,[17] history of acute cellular rejection,[18] and human leukocyte antigen DRB1*08 positivity[18] have all been recently proposed as possible risk factors for rPSC. Other clinical variables have been implicated previously; however, no single risk factor has been definitively or consistently documented as predictive of disease recurrence.
A recent meta-analysis including 14 studies and over 900 patients has revealed an estimated PSC recurrence rate of 17% in patients transplanted for this indication.[19] The true recurrence rate of PSC following OLT remains controversial, and likely underestimated, due to the lack of a diagnostic gold standard or widely accepted diagnostic criteria. Recurrent PSC should be suspected in patients with a previous history of PSC prior to OLT, cholangiographic evidence of biliary strictures, distinct biochemical abnormalities, and classic histopathologic features (i.e. bile duct inflammation, concentric bile duct fibrosis, +/- ductopenia) after all other causes (i.e. chronic rejection, hepatic artery thrombosis, cytomegalovirus infection, etc.) have been sufficiently ruled out.[20,21,22] Distinguishing between rPSC and chronic rejection represents the most significant diagnostic challenge, and often requires serial liver biopsies, because both disorders share many similar features.[23] The major discriminatory features include the patient’s clinical history, the gross appearance of the liver, and histopathologic findings.[23] In rPSC, the liver grossly appears enlarged and fibrotic with notable thickening of the extra-hepatic and large intra-hepatic bile ducts, whereas a more normal-appearing liver is observed in chronic rejection.[23] Additionally, more profound ductopenia (>50% of the portal tracts) and vascular changes are seen in rejection compared with small focal areas of bile duct loss and absence of vascular changes in rPSC.[23]
Prior to transplantation there was no evidence of underlying malignancy in our patient. This case report provides further evidence that de novo post-transplant CCA can develop in patients previously transplanted for PSC. Patients diagnosed with rPSC must be aggressively evaluated for cholangiographic or pathologic evidence of CCA regardless of CCA status prior to initial transplant.
References
1. Wiesner RH, Grambsch PM, Dickson ER, Ludwig J, MacCarty RL, Hunter EB, et al. Primary sclerosing cholangitis: natural history, prognostic factors and survival analysis. Hepatology.1989;10:430–6.
2. Claessen MM, Vleggaar FP, Tytgat KM, Siersema PD, van Buuren HR. High lifetime risk of cancer in primary sclerosing cholangitis. J Hepatol. 2009;50:158–64.
3. Ustundag Y, Bayraktar Y. Cholangiocarcinoma: A compact review of the literature. World J Gastroenterol. 2008;14:6458–66.
4. Xu H, Inagaki Y, Tang W, Guo Q, Wang F, Seyama Y, et al. Elevation of serum KL-6 mucin levels in patients with cholangiocarcinoma. Hepatogastroenterology. 2008;55:2000–4.
5. Li YG, Zhang N. Clinical significance of serum tumour M2-PK and CA19-9 detection in the diagnosis of cholangiocarcinoma. Dig Liver Dis. 2009;41:605–8.
6. Leelawat K, Sakchinabut S, Narong S, Wannaprasert J. Detection of serum MMP-7 and MMP-9 in cholangiocarcinoma patients: evaluation of diagnostic accuracy. BMC Gastroenterol. 2009;9:30.
7. Heneghan MA, Tuttle-Newhall JE, Suhocki PV, Muir AJ, Morse M, Bornstein JD, et al. De-novo cholangiocarcinoma in the setting of recurrent primary sclerosing cholangitis following liver transplant. Am J Transplant. 2003;3:634–8.
8. Landaverde C, Ng V, Sato A, Tabibian J, Durazo F, Busuttil R. De-novo cholangiocarcinoma in native common bile duct remnant following OLT for primary sclerosing cholangitis. Ann Hepatol. 2009;8:379–83.
9. Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BS J, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507– 17; Discussion 517–9.
10. Rosen CB, Nagorney DM, Wiesner RH, Coffey RJ Jr, LaRusso NF. Cholangiocarcinoma complicating primary sclerosing cholangitis. Ann Surg. 1991;213:21–5.
11. Boberg KM, Bergquist A, Mitchell S, Pares A, Rosina F, Broome U, et al. Cholangiocarcinoma in primary sclerosing cholangitis: risk factors and clinical presentation. Scand J Gastroenterol. 2002;37:1205–11.
12. Ghali P, Marotta PJ, Yoshida EM, Bain VG, Marleau D, Peltekian K, et al. Liver transplantation for incidental cholangiocarcinoma: analysis of the canadian experience. Liver Transpl. 2005;11:1412–6.
13. Goss JA, Shackleton CR, Farmer DG, Arnaout WS, Seu P, Markowitz JS, et al. Orthotopic liver transplantation for primary sclerosing cholangitis. A 12-year single center experience. Ann Surg. 1997;225:472–81; Discussion 481–3.
14. Broome U, Olsson R, Loof L, Bodemar G, Hultcrantz R, Danielsson A, et al. Natural history and prognostic factors in 305 swedish patients with primary sclerosing cholangitis. Gut. 1996;38:610–5.
15. Bergquist A, Ekbom A, Olsson R, Kornfeldt D, Loof L, Danielsson A, et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol. 2002;36:321–7.
16. Campsen J, Zimmerman MA, Trotter JF, Wachs M, Bak T, Steinberg T, et al. Clinically recurrent primary sclerosing cholangitis following liver transplantation: a time course. Liver Transpl. 2008;14:181–5.
17. Cholongitas E, Shusang V, Papatheodoridis GV, Marelli L, Manousou P, Rolando N, et al. Risk factors for recurrence of primary sclerosing cholangitis after liver transplantation. Liver Transpl. 2008;14:138–43.
18. Alexander J, Lord JD, Yeh MM, Cuevas C, Bakthavatsalam R, Kowdley KV. Risk factors for recurrence of primary sclerosing cholangitis after liver transplantation. Liver Transpl. 2008;14:245–51.
19. Gautam M, Cheruvattath R, Balan V. Recurrence of autoimmune liver disease after liver transplantation: a systematic review. Liver Transpl. 2006;12:1813–24.
20. Oldakowska-Jedynak U, Nowak M, Mucha K, Foroncewicz B, Nyckowski P, Zieniewicz K, et al. Recurrence of primary sclerosing cholangitis in patients after liver transplantation. Transplant Proc. 2006;38:240–3.
21. Sheng R, Campbell WL, Zajko AB, Baron RL. Cholangiographic features of biliary strictures after liver transplantation for primary sclerosing cholangitis: evidence of recurrent disease. AJR Am J Roentgenol. 1996;166:1109–13.
22. Graziadei IW, Wiesner RH, Batts KP, Marotta PJ, LaRusso NF, Porayko MK, et al. Recurrence of primary sclerosing cholangitis following liver transplantation. Hepatology. 1999;29:1050–6.
23. Demetris AJ. Distinguishing between recurrent primary sclerosing cholangitis and chronic rejection. Liver Transpl. 2006;12:S68–72.